Abstract
BACKGROUND AND PURPOSE: Thickening of the LF is ascribed to buckling due to DSN. Uncertainty exists as to whether this can occur without DSN. Our primary hypothesis was that facet degenerative changes alone, independent of DSN, can thicken the LF. Our secondary hypothesis was that inflammatory changes surrounding degenerative facet joints may incite thickening.
MATERIALS AND METHODS: Fifty-two patients were divided into 1 of 3 groups: group 1 (normal lumbar spine, n = 21), group 2 (LF thickening and FH with normal height of the L4–5 disk, n = 18), and group 3 (LF thickening and FH with decreased height of the L4–5 disk, n = 13). LF thickness measured on axial T1WI at the midpoint of the LF length was compared with that in group 1. Facet joints were evaluated for spurring, joint fluid, and cortical irregularity, indicating facet degeneration. Enhancement of the facet joints and LF thickening were also evaluated (n = 2). The Student t test was used to compare groups.
RESULTS: Normal LF thickness (group 1) was 3.1 mm, whereas LF thickness averaged 4.9 mm in group 2 and 5.3 mm in group 3 (both P < .001). Patients with asymmetric LF thickness showed greater LF thickness on the side with greater FH. There was more LF enhancement on the side with greater facet degenerative disease.
CONCLUSIONS: LF thickening can be secondary to facet degenerative changes, independent of DSN. Inflammatory changes may be an inciting factor for LF thickening.
Abbreviations
- DDD
- degenerative disk disease
- DSN
- disk space narrowing
- FH
- facet hypertrophy
- LF
- ligamentum flavum
- T1WI
- T1-weighted imaging
Thickening of the LF is most commonly attributed to “buckling” of the LF into the spinal canal secondary to loss of intervertebral disk height1–3; however, it is uncertain whether thickening can be present in the absence of DSN. LF thickening has also been associated with FH changes in the absence of DDD,4 as well as other causes such as mechanical stress and physical activity levels.5,6 Furthermore, because >90% of lumbar degenerative disease involves the L4–5 level,4 the uncertainty of the factors responsible for the apparent LF thickening at this level warrants further investigation.
The purpose of this study was to characterize LF thickening in the setting of FH in patients with and without DDD. Therefore, our primary hypothesis was that adjacent facet degenerative changes alone, in the absence of DSN, can cause thickening of the LF. Our secondary hypothesis was that inflammatory changes surrounding the degenerative facet joint may be the inciting etiology of this thickening.
Materials and Methods
This study was approved by our institutional review board. Using imaging data base searching software (Primordial, San Mateo, California), we searched a chronologic MR imaging data base for all attending-verified reports of lumbar spine MR imaging performed and reported at Jackson Memorial Hospital (Miami, Florida), from January 1, 2009, to November 1, 2009. Specifically, we used the following search terms: “ligamentum flavum thickening,” “ligamentum flavum hypertrophy,” “lumbar spine,” “degenerative disk disease,” “disk space height loss,” and “normal lumbar spine MR imaging.”
Initially, 854 reports of lumbar spine MR imaging were identified. Subsequently, each report and its associated MR images were reviewed by a senior radiology resident (F.H.C.) and an experienced board-certified neuroradiologist (R.M.Q.). Studies were excluded if they met the following exclusionary criteria: previous spine surgery, significant disk or metastatic bony/spinal canal disease, intraspinal synovial cysts, scoliosis, and spondylolisthesis at L4–5.
The remaining studies were assigned to 1 of 3 groups: group 1 (normal findings on lumbar spine MR imaging, n = 21), group 2 (LF thickening and FH with normal height of the L4–5 disk, n = 18), and group 3 (LF thickening and FH with decreased height of the L4–5 disk, n = 13).
Disk height at L4–5 was determined by comparison with the L1–4 disk space heights. If all examined levels had comparable heights, without associated osteophyte formation, desiccation, or canal stenosis, the disk was deemed normal. Disk desiccation was determined by signal-intensity loss of the L4–5 disk on sagittal T2-weighted images.
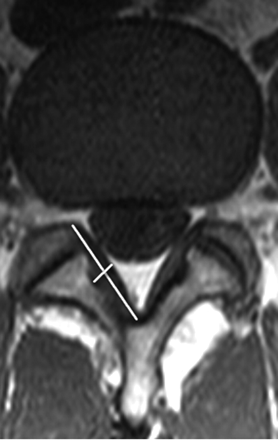
Facet joints were evaluated for the presence of spurring, joint fluid, and cortical irregularity, thereby indicating degeneration. A normal value for LF thickness was established by measuring the thickness at half the length of the LF on axial T1WIs in group 1 (Fig 1), by using the measuring tool on the PACS system. Axial T1WIs were used because they offered better delineation of the LF and facet joints, compared with T2-weighted images. Group 1 had a mean LF thickness of 3.1 mm.
Measurement technique of the LF at the L4–5 level. Thickness of the LF is measured at half the length of the LF on axial T1WI.
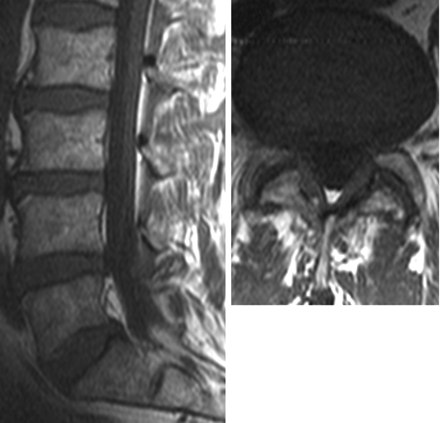
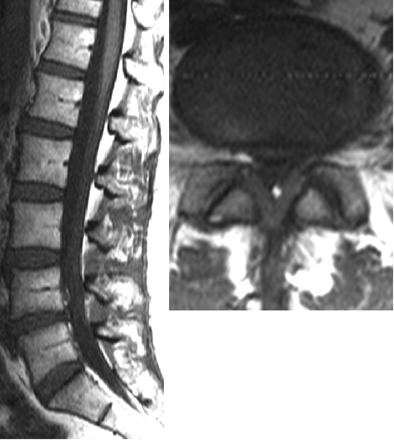
For groups 2 (Fig 2) and 3 (Fig 3), LF thickening was measured on the axial T1WI that was perpendicular to the spinal canal axis and parallel to the laminae, where LF were seen along their entire length. We made 2 measurements: 1) LF width at half its length, and 2) LF width where it appeared the thickest. These values were compared with the mean LF thickness from group 1, 3.1 mm. These 2 measurements were taken to account for any morphologic changes in the LF due to proximity to adjacent FH changes. A Student t test was performed by using LF thickness measurements at half the LF length between the following groups: 1 and 2, 1 and 3, and 2 and 3, with both means and SDs computed.
Sagittal and axial T1WIs of a patient in group 2 show normal L4–5 intervertebral disk height and symmetric bilateral LF thickening and FH changes.
Sagittal and axial T1WIs of a patient in group 3 show both L4–5 intervertebral disk-height loss and bilateral LF thickening and FH changes.
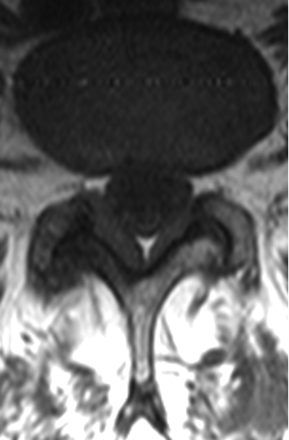
Additionally, the degree of adjacent FH was assessed. Specifically, any asymmetry in LF thickening associated with asymmetric FH was noted (Fig 4). Asymmetry was defined as 1 side having more pronounced FH and LF thickness. In patients with gadolinium-based contrast studies (n = 2), associated inflammatory enhancement of the facet joints and the LF was evaluated as well (Fig 5).
T1WI at L4–5 show asymmetric LF thickening. The LF is thicker on the right side (5.0 mm) compared with the left (3.9 mm); FH is also ipsilaterally worse on the right side relative to the left.
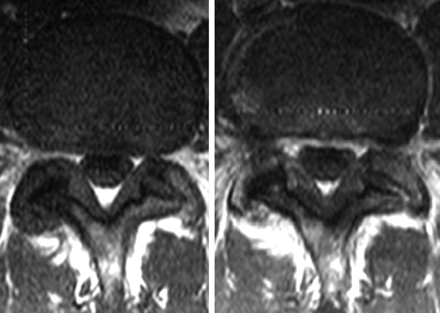
Pre- (left) and postcontrast (right) axial T1WIs at L4–5, show asymmetric thickening of the LF and FH, with subtle enhancement of the LF adjacent to the hypertrophied facet joint. Subtle enhancement of the lateral aspect of the left facet joint is also seen.
Results
Evaluation of 21 lumbar spine MR images (group 1) with normal findings yielded an average half-length LF thickness of 3.1 ± 0.8 mm. The average half-length LF thickening within the remaining groups was 4.9 ± 1.0 (group 2) and 5.3 ± 0.7 mm (group 3) (Figs 2 and 3). These measurements were significantly (P < .001) greater than the width of the normal LF (group 1). The average maximal LF thickness was 5.6 ± 1.0 mm (group 2) and 5.8 ± 0.9 mm (group 3).
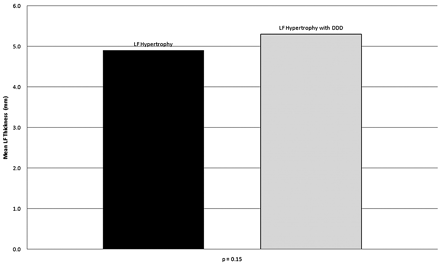
Additionally, no statistically significant difference in LF thickening was made at half-length (P = .15) (Fig 6) or maximal width (P = .40) in groups 2 and 3.
Mean LF thickness of group 2 (4.9 mm) and group 3 (5.3 mm) at half-length of the LF at L4–5. No statistically significant difference in LF thickness was seen at half-length between groups 2 and 3 (P = .15).
Patients with asymmetric LF thickening showed greater LF thickness on the side with worse facet degenerative disease, as subjectively evaluated (Fig 4). Also, postcontrast studies showed more enhancement of both the facet joint and the LF on the side with greater facet degenerative disease (Fig 5).
Discussion
There is uncertainty as to whether apparent thickening of the LF is due solely to loss of disk space height and subsequent LF buckling or whether LF thickening may be present in the absence of an abnormal disk space. To address this issue, we examined 2 cohorts of patients (groups 2 and 3). Both groups had LF thickening and FH; however, only group 3 also had intervertebral disk-height loss at L4–5.
Besides the often-observed buckling of the LF, reports in the literature also ascribe LF thickening to other factors such as mechanical stress and physical activity levels.5,6 Increasing patient age has been shown to correlate with LF thickening at the L4–5 level,6,7 presenting as young as 30 years of age in 1 recent study8; however, Safak et al9 have recently found no such association.
Additionally, even though buckling of the LF secondary to intervertebral disk-height loss has often been cited,1–3 LF thickening has also been associated with FH changes,4 which, in association with other findings, contributed to lumbar spinal stenosis, sometimes in the absence of DDD.8 Our results not only support these observations and our primary hypothesis but suggest that there may also be an independent relationship between intervertebral disk height and LF thickening. This is especially true in the few contrast-enhanced studies that showed asymmetric FH associated with ipsilateral LF thickening and enhancement.
In patients with spinal stenosis, Park et al5 found a mean thickness of the LF of 4.44 mm, thicker than the LF thickness in the control group (2.44 mm). Other studies have shown normal thicknesses of the LF, ranging from 1.8 to 5 mm.4,9–11 Our results for normal LF thickening, showing a mean of 3.1 mm, are similar to the findings in these studies.
Moreover, our findings and observations strengthen the secondary hypothesis of this study that inflammatory changes may be an inciting factor for LF thickening. Histologic studies have found that fibrocartilaginous transformation provoked by collagen proliferation and ossification can lead to LF thickening.6,12 Age-related fibrosis or decrease in the elastin-to-collagen ratio, in combination with biomechanical stress, has been reported to lead to thickening of the LF.5–7 Pathologic studies have revealed leakage of inflammatory cytokines from degenerating facet joints, implicating these molecules as causes of both LF thickening and pain generation in the adjacent nerve fibers.13,14 Additionally, Sairyo et al15 have found LF thickening to be a result of scarring from inflammation, prompted by mechanical stress-induced tissue damage.
As a corollary, we also found that in cases of asymmetric LF thickening, both the thickness and postcontrast enhancement were greater on the side harboring the more severe facet degeneration (Figs 4 and 5). This finding implies a spatial and causal relationship between facet degenerative changes and LF thickening.
Careful and systematic evaluation of facet joints and the LF is important. Specifically, recognition of the correlation and possible causal relationship between FH and LF thickening can facilitate detailed and relevant evaluation of the cause of lumbar spinal stenosis. Particularly in patients with normal L4–5 intervertebral height, the LF and facet joints should be assessed for symmetric or asymmetric concordant disease. In patients with lumbar pain with facet joint and LF enhancement, both infection and severe degenerative disease should be kept in mind. Furthermore, in the presence of significant LF thickening and FH, gadolinium-based contrast administration could be considered to determine if the facet disease is a cause of pain generation.
In addition, facet degenerative changes and LF disease should be particularly noted in the interpreting radiologist's final report, even when the intervertebral disk spaces appear normal. This is especially important when the referring physician is considering an intervention for relief of pain and/or neurologic symptoms. Identifying “pure” LF pathology may mitigate the need for extensive spinal surgery and prompt the surgeon to undertake a minimally invasive technique.10 In addition, the rich bilevel innervation of the posterior elements by the posterior ramus of the spinal nerve can produce referred pain.4 With radicular distribution at the 2 adjacent ipsilateral facet levels, detailed identification of the pathologic facet and LF levels can help explain the patient's symptoms in such cases. Drug therapy with anti-inflammatory agents may help reduce or control LF thickening because it has been found to be a scarring phenomenon.15
This study had several limitations. Age and sex were not considered when stratifying patients in 1 of 3 groups because our goal was simply to assess whether LF thickening can be seen independent of DSN. The analysis of disk space heights and FH changes was subjective; the study was not prospective.
Future studies could include assessment of normal and abnormal LF thickness as it relates to age and sex and/or could assess LF enhancement in a greater number of subjects.
Conclusions
Although DDD is a major factor in the morbidity associated with lumbar spinal disease, our investigation supports our primary hypothesis that adjacent facet degenerative changes alone, in the absence of DSN, can cause thickening of the LF. Moreover, postcontrast images of LF thickening and FH support our secondary hypothesis that inflammatory changes surrounding the degenerative facet joint may be the inciting etiology of this thickening.
Footnotes
-
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, May 15–20, 2010; Boston, Massachusetts.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received March 31, 2010.
- Accepted after revision May 12, 2010.
- Copyright © American Society of Neuroradiology