Abstract
BACKGROUND AND PURPOSE: Extracranial-intracranial (ECIC) bypass grafts have been assessed postoperatively by various neuroradiologic techniques. The aim of this prospective study was to evaluate postoperative changes in ECIC bypass graft by using superficial temporal artery duplex ultrasonography (STDU). Furthermore, this study assessed the ability of STDU to predict cerebrovascular reserve capacity (CVR).
MATERIALS AND METHODS: Forty-five consecutive patients who underwent ECIC bypass procedure for atherosclerotic internal carotid artery occlusion were enrolled in this prospective study. All patients underwent single-photon emission CT and STDU preoperatively, 14 days after, 3 months after, 1 year after, and 2 years after ECIC bypass.
RESULTS: The diameter and flow velocities of the ipsilateral superficial temporal artery (STA), and regional cerebral blood flow (rCBF) showed increase during the first 2 weeks and then remained stable, whereas CVR showed a constant improvement up to 2 years after surgery. The STA diameter and mean STA flow velocity correlated significantly with CVR at 1 year after surgery (r 2 = 0.1232 and r 2 = 0.08716, respectively; P < .05). A cutoff value of 1.8 mm STA diameter was determined as the most reliable value to predict CVR greater than 10% at 1 year after surgery. The positive predictive value was calculated as 96.6%, the negative predictive value as 43.8%, the sensitivity as 75.7%, the specificity as 87.5%, and the likelihood ratio as 6.056.
CONCLUSIONS: ECIC bypass grafts can be assessed postoperatively in a noninvasive fashion with STDU. This technique provides information regarding patency as well as quantitative assessment of bypass function. Moreover, STDU is useful to predict CVR improvement.
Since the introduction of extracranial-intracranial (ECIC) bypass in 1969,1 the procedure has been used for the effective treatment of internal carotid artery occlusion and middle cerebral artery stenosis.1–4 Although 1 international randomized trial reported that ECIC bypass surgery was not effective to prevent stroke, that study may have been limited by its broad inclusion criteria.5 By contrast, a randomized trial of strictly selected patients with severe cerebral hemodynamic failure has just been finalized in Japan (Japan ECIC bypass trial: JET study; unique trial number C000000144).6 Previous studies suggested that improvement in blood flow via ECIC bypass correlated with presurgical cerebral hemodynamics.7–9 Indeed, many authors demonstrated that patients with impaired vasodilatory ability to a physiologic stimulus were at very high risk for subsequent stroke if not treated.10–13 Such hemodynamic conditions have now been recognized as reduced cerebrovascular reserve capacity (CVR).14–17
Catheter- and MR-based angiography are typically used to evaluate the postoperative bypass patency.1,5,18,19 Because such anatomic grading systems assess the patency alone,20 they are not adequate tools to predict the blood flow arriving to the brain and, consequently, the outcome. Therefore, other modalities are needed to assess the function of ECIC bypass. Although the short-term effects of ECIC bypass on cerebral hemodynamics have been investigated with various neuroradiologic techniques, including positron-emission tomography (PET),21–23 single-photon emission CT (SPECT), and xenon-enhanced CT scanning,8,16,22–24 these modalities are complicated, expensive, time consuming, and invasive because they expose patients to radiation, making them ill suited for use in the outpatient setting or for long-term routine follow-up of ECIC bypass. As a result of the unavailability of simple assessment techniques, only a few reports have been published on postoperative maturation of bypass grafts.25,26 For example, Murata et al26 used near-infrared spectroscopy to demonstrate that ECIC bypass maintained cerebral blood oxygenation at 1 year postoperatively, which suggests that the superficial temporal artery (STA) flow increased gradually for a certain period postoperatively. We recently described the usefulness of STA duplex ultrasonography (STDU) in the assessment of short-term perioperative changes in STA flow velocity and diameter after ECIC bypass.27,28 The ipsilateral STA mean blood flow velocity is a highly sensitive parameter to predict regional cerebral blood flow (rCBF) in the ipsilateral middle cerebral artery (MCA) territory at 14 days after ECIC bypass in patients with internal carotid artery occlusion or middle cerebral artery stenosis.
The goal of our study was to perform a similar analysis on a longer (2 year) time scale. Internal carotid artery occlusive lesions were assessed, and the hypotheses were proposed: 1) ECIC bypass continues to develop both morphologically and functionally after surgery, resulting in improving cerebral hemodynamics over time; and 2) STDU provides the ability to noninvasively assess both the patency and the function of ECIC bypasses and to easily predict cerebral hemodynamics even in the outpatient setting.
Materials and Methods
Patient Population
Forty-five consecutive patients who underwent ECIC bypass procedure (ie, anastomosis of the STA and MCA) for internal carotid artery occlusion in our institution from May 2001 to November 2006 were enrolled in this prospective study. This study was approved by the Ethical Committee of our institute. All patients gave informed consent to undergo cerebral angiography and the ECIC bypass procedure. In accordance with the entry criteria for the JET study,6 the following criteria were adopted as indications for ECIC bypass: rCBF of the ipsilateral MCA less than 32 mL/100 g/min (corresponding to 80% of normal value), and a CVR less than 10% in a quantitative SPECT study with a acetazolamide (ACZ) challenge. Angiographic confirmation of bypass patency was obtained at 14 days after surgery in all cases. All patients underwent MR angiography, SPECT, and STDU before, 14 days after, 3 months after, 1 year after, and 2 years after ECIC bypass.
Surgery
All STA-MCA anastomotic procedures were performed by the same neurosurgical team. Anesthesia included fentanyl citrate, thiamylal sodium, and propofol. Single STA-MCA anastomosis was performed in the patients whose anterior or posterior STA branch was not suitable to harvest or who had severe arteriosclerosis obliterans preoperatively. Blood pressure was controlled to a target of less than 150 mm Hg systolic and 90 mm Hg diastolic in all patients with use of nitroglycerin and/or diltiazem for 7 days after the surgery.
SPECT
The SPECT apparatus consisted of the PRISM 2000X device (2-head SPECT system; Picker, Cleveland, Ohio), and iodine 123 N-isopropyl-p-[123I]-iodoamphetamine (123I-IMP) was used as the tracer. An elliptical region of interest, more than 16 cm2 in size, was placed in the cortical area in the MCA territory of each side. Areas of infarct, if present, were carefully excluded from the region of interest, if possible. We measured the resting rCBF values quantitatively by using autoradiography.29 The rCBF values were also measured after the ACZ challenge test. We expressed CVR using the following equation: CVR = (post-ACZ rCBF-resting rCBF)/resting rCBF.
STDU
An HDI-5000 (Philips, Bothell, Wash) with a 12- to 5-MHz linear array transducer was used for STDU. Subjects were examined by well-trained and experienced sonographers. The sonographers were technicians who had been educated in basic sonography technology and human anatomy. In addition, they received 3 months to 1 year of training, including use of the sonography system, reading procedures, and quality-control programs. For the examination of STA, each patient was examined in the supine position with the patient's head facing the contralateral side. The transducer was placed in the temporal region in close approximation to the external auditory meatus, and STA was displayed on the B-mode image with color Doppler. The diameter of the bilateral STA was measured. On the longitudinal scans, the sample volume was set within the STA at a point proximal to its bifurcation. The incident angle between the STA and the Doppler beam was kept at 60° or less. Range-gated pulsed Doppler sonography was used to measure the blood flow velocity of the STA. We measured the absolute value of peak-systolic, end-diastolic, and mean flow velocity of bilateral STAs by using the maximal frequency envelope.
MR Imaging
We performed MR imaging preoperatively, 2 weeks, 3 months, 1 year, and 2 years after surgery by using a 1.5T whole-body MR imager (Magnetom Symphony, Siemens, Erlangen, Germany; and Signa Infinity Excite, GE Healthcare, Milwaukee, Wis). In all patients, the diameter of STA at 1 year after surgery was measured at just proximal to the surgical bone defect of the temporal bone on thin-section enhanced axial MR imaging.
Data Analysis
Changes in parameters of STDU and SPECT were observed, and the relationships between the parameters were investigated. To analyze postoperative change in STDU and SPECT parameters, we obtained a dataset from 30 patients who were observed for 2 years after surgery. For unpaired t test and linear regression analysis, we obtained a dataset from 45 patients who were observed for 1 year after surgery. The Friedman test with post hoc Dunn test was used to assess differences in STA flow velocities, STA diameter, rCBF, or CVR before and after surgery. The unpaired t test was used to assess differences in STA diameter or STA flow velocity according to CVR. Linear regression analysis and the Pearson correlation coefficient were used to evaluate the correlation between STA diameter of MRA and STDU, and between changes in CVR and the mean STA flow velocity or STA diameter. Cutoff values were defined for STA diameter. Sensitivity, specificity, and the likelihood ratio were calculated to predict postoperative CVR higher than 10%. In all procedures, a P value of less than .05 was considered to represent statistical significance.
Results
Patient Population
Thirty-five men and 10 women (mean age ± SD, 62 ± 7.8 years; age range, 45–78 years) were included. With respect to cerebrovascular risk factors, hypertension was present in 32 patients (71%), diabetes mellitus in 21 (47%), hypercholesterolemia in 25 (56%), smoking in 24 (53%), and chronic alcohol use in 28 (62%). Initial symptom was transient ischemic attack in 16 patients (36%) and brain infarction in 25 patients (56%). Two patients had previous brain infarction. Eighteen patients underwent single STA-MCA anastomosis, and 27 patients underwent double STA-MCA anastomosis. Of these, 30 patients were observed for more than 2 years after surgery. Complications were not observed for any patients undergoing the STA-MCA anastomosis procedure. There were no neurologic symptoms or new ischemic lesions on the MR imaging during the observation period. STA diameter of MRA correlated with that of STDU at 1 year after surgery (r2 = 0.1208; P < .05).
Postoperative Changes in STDU Parameters
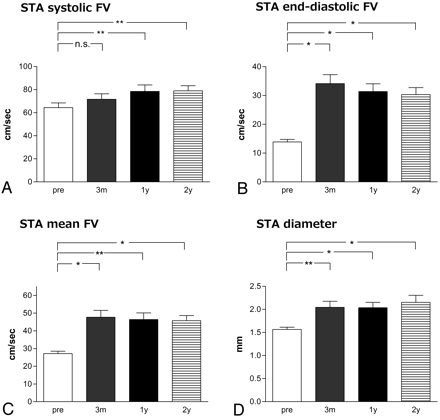
Flow velocities of the ipsilateral STA were significantly higher at 14 days after ECIC bypass compared with before bypass (27.2 ± 6.2 cm/s preoperatively vs 57.1 ± 23.8 cm/s 14 days after surgery, P < .0001 in mean flow velocity; 13.9 ± 4.2 cm/s preoperatively vs 40.2 ± 18.0 cm/s 14 days after surgery, P < .001 in end-diastolic flow velocity; 64.3 ± 18.7 cm/s at preoperatively vs 91.5 ± 33.7 cm/s 14 days after surgery, P < .001 in systolic flow velocity). Furthermore, these values remained stable between 3 months and 2 years after surgery (Fig 1). The diameter of the ipsilateral STA was also larger at 14 days after ECIC bypass compared with before bypass (P < .0001), and these values remained stable between 3 months and 2 years after surgery. There was no statistical difference in STDU parameters between single and double anastomosis at 1 year after surgery (data not shown).
Postoperative changes in systolic (A), end-diastolic (B), and mean (C) STA flow velocity (FV), and diameter or STA (D). *P < .01, **P < .05, n.s. = not significant.
Postoperative Changes in SPECT Parameters
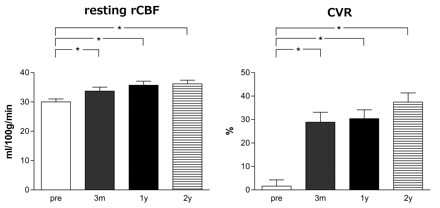
The resting rCBF and CVR of the ipsilateral MCA territory were significantly higher at 14 days after ECIC bypass compared with before bypass (P < .0001 in each). Resting rCBF remained stable between 3 months and 2 years after surgery (Fig 2). By contrast, CVR continued to increase gradually until 2 years after surgery (1.6 ± 13.7% preoperatively, 22.2 ± 14.9% 14 days after surgery, 27.4 ± 22.2% 3 months after surgery, 29.0 ± 20.0% 1 year after surgery, and 36.1 ± 20.8% 2 years after surgery). There was no statistical difference in SPECT parameters between single and double anastomosis 1 year after surgery (data not shown).
Postoperative changes in resting rCBF (left) and CVR (right). *P < .01, **P < .05.
Correlation between STDU Parameters and SPECT Parameters
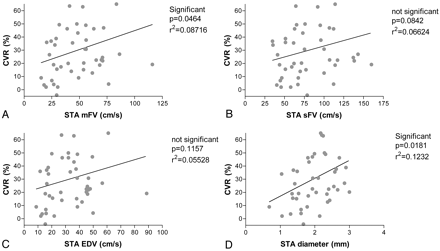
Among the 3 parameters of STA flow velocity (Table 1), the mean STA flow velocity had the strongest correlation with CVR (r2 = 0.08716; P < .05; Fig 3). STA diameter also correlated with CVR (r2 = 0.1232; P < .05). Both correlations were stronger at the 1-year time point than at the 3-month time point (data not shown). Resting rCBF did not correlate with any parameters of STDU.
Correlation between STDU parameters and SPECT parameters at 1 year after surgery. Mean STA flow velocity (mFV) and CVR (A), systolic STA flow velocity (sFV) and CVR (B), end-diastolic STA flow velocity (EDV) and CVR (C), and STA diameter and CVR (D).
Correlation between STDU parameters and CVR at different time point after surgery
Predictive Values of STA Diameter for CVR Greater than 10%
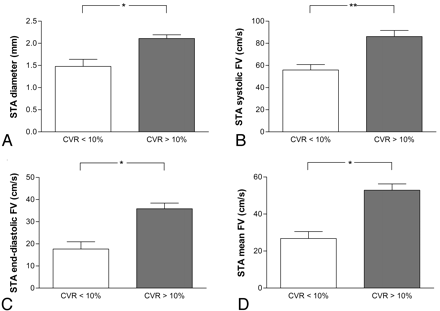
At 1 year after ECIC bypass, 82% of patients demonstrated CVR greater than 10%. The Student t test revealed a statistically significant difference in STA diameter between patients with high CVR (>10%; n = 37) and with low CVR (<10%; n = 8; P < .01) 1 year after surgery (Fig 4). This difference was more significant than at 14 days (P < .05) or 3 months after surgery (P < .05). The Student t test also revealed a statistically significant difference in STA flow velocities between patients with high CVR and with low CVR at 1 year after surgery (P < .05 in systolic flow velocity, P < .01 in end-diastolic flow velocity, P < .001 in mean flow velocity). As shown in Table 2, a cutoff value of 1.8 mm was detected as the most reliable value to predict CVR greater than 10%. The positive predictive value was calculated as 96.6%, the negative predictive value as 43.8%, the sensitivity as 75.7%, the specificity as 87.5%, and the positive likelihood ratio as 6.056.
Difference in diameter of STA (A), in systolic flow velocity (B), end-diastolic flow velocity (C), or mean flow velocity (D) between patients with low CVR (<10%; n = 8) and with high CVR (>10%; n = 37) at 1 year after surgery.
Predictive values for cerebrovascular reserve capacity higher than 10% at different cutoff values of diameter of STA at 1 year after ECIC bypass surgery
Discussion
Our study demonstrated that STDU can easily and serially confirm the patency and long-term changes in bypass morphologic features and function after ECIC bypass. Furthermore, STA bypass grafts were stable at least until 2 years after surgery.
Bypass failure occurs at an average of 2.7 years after surgery.30 However, modalities for long-term postoperative follow-up of bypass grafts function (eg, PET and SPECT) are not cost effective for serial use in the outpatient setting. Amin-Hanjani et al31 reported that quantitative MR angiography could directly measure flow in milliliters per minute in the vessels of interest. Our study demonstrated that STDU is likely of similar usefulness. In contrast to SPECT, MR imaging, or DSA, STDU is noninvasive, is available at the bedside or outpatient clinic, and is well suited for serial examination without any contraindications. Arakawa et al32 previously described the usefulness of STDU in the evaluation of STA flow velocity. They indicated that the end-diastolic flow velocity ratio of the operated STA to the contralateral STA is a highly sensitive parameter to evaluate the extent of the bypass flow.
Previous studies have described short-term data for STDU in the perioperative evaluation of ECIC bypass grafts.27,28 For example, Inoue et al28 demonstrated that mean STA flow velocity significantly correlated with resting rCBF at 14 days after ECIC bypass, whereas mean STA flow velocity and STA diameter did not correlate with CVR. In our study, there was no correlation between STDU parameters and resting rCBF, but STDU parameters correlated with CVR at 1 year after surgery. The mechanism underlying the disappearance of the correlation between mean STA flow velocity and resting rCBF at 1 year after ECIC bypass remains unclear. In the postoperative developing process of the bypass, there might be a limitation in the dilation of the STA, and the increase in rCBF might depend only on an increase in the STA flow velocity, whereas rCBF might be maintained constantly not only by the STA flow velocity but also by the dilation of the STA after establishment of autoregulation. A possible reason for the lack of the correlation between STDU parameters and CVR at 14 days after ECIC bypass is that the STA was maximally dilated in the immediate postoperative period because of preoperative impairments in cerebral hemodynamics; in other words, the vasodilatory ability of the STA had not yet recovered. Our study focused on CVR rather than on resting CBF because postoperative improvements in CVR may result from newly established collateral vessel from the extracranial to the cerebral circulation, especially in view of the substantial anastomotic filling of cortical vessels.8 Although the underlying mechanism remains unclear, results from our study suggest that the matured STA may have the ability to respond to physiologic stimuli.
Another goal of this study was to assess the ability of STDU to predict CVR reserve and address whether this technique may by an adequate replacement for SPECT for the serial follow-up of bypass graft function. Many investigators have reported that patients with impaired CVR are at particularly high risk for subsequent stroke.10–13 Our study demonstrated that an STA diameter of 1.8 mm was a valuable cutoff value for the prediction of reserved cerebrovascular capacity. In the population in our study, all preoperative STA values were less than 1.8 mm. However, the STA in some patients with transdural anastomosis (ie, Moyamoya disease) are large, even preoperatively. In such patients, this cutoff value may not be reliable. Regardless, for clinical use in the general population, a 100% positive predictive value at 2.2-mm STA diameter and a 100% negative predictive value at 1.0-mm STA diameter are likely useful.
Our study had some drawbacks. We did not measure flow velocities and the diameter of STA before and after stimulation challenge. It would be helpful to confirm the theory that the STA was able to dilate in response to stimuli. In addition, we did not perform an outcome analysis because no patients demonstrated any cerebral ischemia during the observation. Additional follow-up is needed to clarify whether this technique can be used to predict adequacy of flow for the prevention of future strokes.
Conclusions
ECIC bypass grafts can be assessed postoperatively in a noninvasive fashion with use of STDU. This technique provides information regarding patency as well as quantitative assessment of bypass function. Moreover, a cutoff value of 1.8-mm STA diameter is useful to predict CVR improvement.
References
- Received October 20, 2008.
- Accepted after revision November 20, 2008.
- Copyright © American Society of Neuroradiology