Abstract
SUMMARY: Epilepsy is a chronic disorder affecting approximately 1% of the population of the world. Approximately one third of patients with epilepsy remain refractory to medical therapy. For these patients, surgery is a curative option. In order for surgery to be considered, precise localization of the structural abnormality is needed. When MR imaging findings are normal, more sensitive techniques such as positron-emission tomography (PET) can help find the abnormality. Combining MR imaging and PET information increases the sensitivity of the presurgical evaluation. In this review, we discuss the clinical applications of coregistration of [18F] fluorodeoxyglucose (FDG)-PET with MR imaging for medically refractory epilepsy. Because FDG-PET/MR imaging coregistration has been a routine component of the presurgical evaluation for patients with epilepsy at our institution since 2004, we also included cases from our data base that exemplify the utility of this technology to obtain better postsurgical outcomes.
Epilepsy is a chronic neurologic disorder that affects approximately 1% of the population of the world with an incidence of 70 per 100,000 persons per year.1
About one third of patients with partial epilepsy do not respond to pharmacotherapy.2 In these patients, epilepsy surgery is a potentially curative option.3 Identification of structural lesions is vital in the presurgical evaluation. MR imaging is a powerful tool to identify the lesion causing epilepsy. Recent advances in MR imaging techniques have increased the sensitivity of detecting subtle structural abnormalities. However, despite these advancements, MR imaging still fails to reveal any apparent abnormality in approximately 20% of the patients with medically refractory epilepsy.4
Patients with epilepsy and normal MR imaging findings have long been the subject of intense research. Other techniques such as positron-emission tomography (PET), ictal single-photon emission tomography, and proton spectroscopy have been used to depict abnormalities that may indicate the epileptogenic zone. These methods can show the laterality of the lesion, but the precise localization is still difficult to obtain due to lack of anatomic landmarks. Furthermore, patients with partial seizures demonstrated on ictal scalp electroencephalography (EEG) with negative MR imaging findings will often undergo invasive intracranial monitoring to localize the epileptogenic zone. Thus, these techniques may not only fail to localize the lesions but may also fail to deter more invasive studies.
However, [18F] fluorodeoxyglucose–positron-emission tomography (FDG-PET) is highly sensitive in localizing epileptic foci and is able to provide information complementary to MR imaging.5 Thus, coregistration of MR images and FDG-PET images may enhance presurgical management of refractory epilepsy. This has already been shown to be true for certain underlying causes of medically refractory epilepsy.6,7 Additionally, FDG-PET/MR imaging coregistration has already been the subject in 1 clinical study,8 which demonstrated favorable postsurgical outcomes in 86% of patients with refractory epilepsy with the application of coregistered imaging. Compared with this, in studies of patients in whom the use of anatomic and functional multimodality imaging coregistration was either absent or very limited for presurgical planning, only 30%–76% of patients had favorable outcomes at a similar follow-up period.8–10 Reasons for this lower rate of favorable outcome without coregistered image-guided surgery include difficulty in delineating the epileptogenic zone and in correlating it with relevant structural anatomy for surgical planning.8
In this review, we briefly describe how presurgical evaluation for medically refractory epilepsy and FDG-PET/MR imaging coregistration is performed at the University of California, Los Angeles (UCLA). We then aim to describe the clinical applications of MR imaging and FDG-PET for presurgical evaluation of refractory epilepsy due to numerous common underlying etiologies, including mesial temporal sclerosis (MTS), focal cortical dysplasia (FCD), epileptogenic tumors, and tuberous sclerosis complex (TSC). For each underlying condition, we will additionally describe and provide examples of how the application of FDG-PET/MR imaging coregistration can improve management of these patients and their prognoses. In doing so, we hope to encourage further clinical study of the use of FDG-PET/MR imaging coregistration for presurgical evaluation of medically refractory epilepsy.
Presurgical Evaluation and FDG-PET/MR Imaging Coregistration for Medically Refractory Epilepsy at UCLA
Presurgical evaluation for medically refractory epilepsy at UCLA entails a multimodality approach, which includes history, physical examination, interictal and ictal scalp EEG video recordings, MR imaging, FDG-PET, and FDG-PET/MR imaging coregistration. The resulting information is then discussed in a multidisciplinary conference to determine the best management for each patient. If necessary, as in patients in whom the initial evaluation results in conflicting or insufficient information, additional studies are performed. These include magnetic source imaging, neuropsychological assessment, the Wada test, and sometimes invasive depth-electrode or grid placement. If surgery is pursued, intraoperative electrocorticography, motor-sensory mapping with somatosensory evoked potentials, and language localization with direct cortical stimulation may be performed.
MR imaging is performed on a 1.5T Sonata scanner (Siemens Medical Systems, South Iselin, NJ). FDG-PET scans are performed by using a CTi/Siemens whole-body positron tomography system (ECAT EXACT or ECAT HR+; Siemens/CTi, Knoxville, Tenn). FDG-PET/MR imaging coregistration is performed by using Fusion 7 software (Mirada, Oxford, England) with a Vitrea 3D workstation (Vital Images, Minnetonka, Minn) as previously described.7 Automatic alignment of FDG-PET and MR images is performed by using the software without fiducial markers. Fused images are displayed as multicolored images with each color change corresponding to approximately a 15% difference in 18F-FDG uptake. Fused images are created with color-coded grading with white as the highest metabolism followed by red, orange, yellow, green, blue, and gray as lowest. Areas of hypometabolism or hypermetabolism are determined on the basis of the asymmetry of 18F-FDG uptake compared with the contralateral structures. In cases in which the visual asymmetry is more subtle, we supplement the visual assessment by using standard uptake value ratios to define whether an area is abnormal. We accept a difference of 10% as significant.7 The MR imaging and PET images are interchangeable by using the fusion program, allowing areas of abnormal 18F-FDG uptake to be directly correlated with possible abnormalities on structural MR imaging.
Cases described in this review are previous patients at UCLA who were discussed in the multidisciplinary conferences between January 2004 and December 2007. Cases were reviewed individually, and the most relevant examples were selected. Informed consent was obtained to use clinical, neuroimaging, and histopathology data for research purposes. The UCLA Institutional Review Board approved this work.
MTS
Mesial temporal lobe epilepsy (MTLE) is the most frequent form of partial epilepsy. It is estimated that 60%–75% of patients with MTLE have MTS, which is characterized by neuronal loss and gliosis of mesial temporal structures, including the hippocampus, amygdala, and parahippocampal gyrus.11 The epilepsy associated with MTS is most often medically refractory, but surgical intervention is relatively effective with 48%–84% of patients being seizure-free after surgery.12 This relatively high success rate of surgery for the epilepsy associated with MTS emphasizes the importance of the presurgical evaluation.
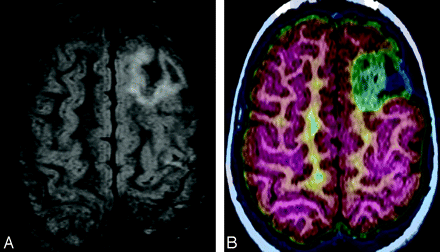
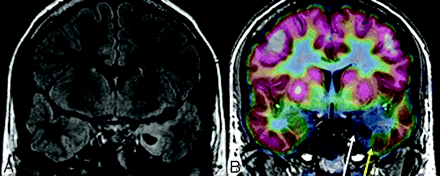
The diagnosis of MTS can be confirmed with MR imaging, with a sensitivity of 81% compared with pathology as a standard.13 Fluid-attenuated inversion recovery (FLAIR) sequencing can further increase detection rate of MTS. However, there are many situations in which MR imaging findings are subtle or absent or are inconsistent with clinical semiology or EEG findings. If multifocal abnormalities are present, they may result in discordant EEG, MR imaging, and/or semiology.14 Furthermore, if hippocampal sclerosis is severe, as in so called “burned-out hippocampus” syndrome, rapid contralateral spread of ictal discharges can result in falsely lateralizing EEG and thus discordance between EEG and MR imaging.15 In patients with MTS, postsurgical seizure control is worse when MR imaging and EEG findings are discordant.16 FDG-PET can complement MR imaging (Fig 1).5,14
MTS in a 31-year-old woman. A, FDG-PET image was read as having normal findings. B, However, the FLAIR image shows some question of increased T2 signal intensity in the right hippocampus. C, FDG-PET/MR imaging coregistration demonstrates subtle hypometabolism of the right mesial temporal structures. Because neurocoginitive testing also demonstrated impaired function of right mesial temporal structures, right anteromedial temporal lobectomy and hippocampectomy were performed. FDG-PET/MR imaging coregistration facilitated localization of the metabolically abnormal area that was subtle when read by FDG-PET alone.
FDG-PET detects hypometabolism in approximatley 80% of patients with unilateral MTLE.17 The hypometabolic zone often exceeds the mesial temporal lobe and involves the ipsilateral temporal neocortex, thalamus, basal ganglia, and frontal regions. Patients who undergo anterior temporal resection will often show normalization of glucose metabolism in the ipsilateral frontal lobe and thalamus. Furthermore, FDG-PET can provide prognostic data in regard to the epilepsy because interictal hypometabolism localized in the temporal area predicts favorable postsurgical outcome, whereas interictal hypometabolism extending outside the temporal lobe predicts worse postsurgical outcome.18 Worse prognosis has been also been found to be associated with less surgical resection of the area of hypometabolism found on FDG-PET and discordance between FDG-PET and MR imaging.18,19 Additionally, FDG-PET may demonstrate bitemporal interictal hypometabolism and thus guide management toward palliative surgery.20
FCD
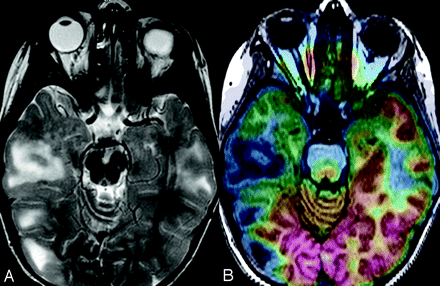
FCD is the most common malformation of the cortical development in surgically treated patients with medically refractory epilepsy. Histopathologically, FCD is divided into 2 subtypes: type I and type II.21 FCD type II is associated with dysmorphic or dysplastic neurons often with balloon cells. MR imaging of FCD type II shows abnormal gyral and sulcal patterns and blurring of the gray-white matter junction (Fig 2). FCD type I is often associated with immature neurons in the white matter. MR imaging of FCD type I shows subtle gray-white matter junction blurring and signal-intensity changes predominantly in the white matter and atrophy. MR imaging of FCD type I has been challenging, despite improvements in recent MR imaging techniques, because it usually yields only normal or subtly abnormal MR imaging findings (Fig 3). Studies have demonstrated that only approximately 30%–70% of patients with type I FCD have positive findings on MR images, compared with 80%–100% of those with type II FCD. Many type I FCD MR images are read as having normal findings.22–25
FCD type II in a 5-year-old girl. A, T2-weighted MR image demonstrates hyperintensity in the left frontal white matter suggestive of FCD. B, With FDG-PET/MR imaging coregistration, a focal area of hypometabolism is seen correlating to the lesion. Pathologic findings of the surgical resection specimen were consistent with FCD type II.
FCD type I in a 3-year-old boy. A, T2-weighted MR image shows questionable hyperintensity and atrophy in the left temporal pole. B, FDG-PET/MR imaging coregistration shows that this area corresponds to an area of mild hypometabolism. After a left lateral temporal lobe resection, pathologic findings were consistent with FCD type I.
In comparison, FDG-PET can identify FCD despite normal MR imaging findings.7,26 One study found that MR imaging detected 83% of severe FCD, whereas FDG-PET detected 90%.26 However, for subtle FCD, MR imaging identified only 13% compared with 86% with FDG-PET. This suggests that FDG-PET is more sensitive than MR imaging and that it is particularly useful when MR imaging findings are normal. One study has already demonstrated that the use of FDG-PET/MR imaging coregistration cannot only improve detection of FCD, particularly in cases of Type I FCD, but also reduce the need for invasive studies.7
FDG-PET/MR imaging coregistration may also facilitate surgical planning by allowing the superimposition of metabolic abnormalities on particular gyri or sulci. There are cases in which complete excision according to MR imaging did not achieve seizure-free outcome due to persistent MR imaging−occult pathology.27 The additional use of FDG-PET may help to disclose these occult areas. In fact, for FCD, the boundaries of the cortical abnormality are usually larger on FDG-PET imaging than on MR imaging.26 Therefore FDG-PET/MR imaging coregistration may provide useful additional information and may be helpful both in terms of surgical planning and surgical outcome in patients with FCD.7,26
Epileptogenic Tumors
Tumors are a major cause of medically refractory epilepsy, accounting for 10%–30% of primary pathology in these patients.28,29 Such tumors include dysembryoplastic neuroepithelial tumors (DNET), gangliogliomas, astrocytomas, and oligodendrogliomas.30 MR imaging has been established as an essential tool for the diagnosis and localization of these tumors related to refractory epilepsy.31
FDG-PET/MR imaging coregistration can also be helpful for abnormalities outside the tumor found in association with these epileptogenic tumors. FCD, most commonly type I, is found with epileptogenic tumors, particularly with the glioneural tumors: gangliogliomas and DNET.32 One study demonstrated that almost half of patients with refractory epilepsy with gangliogliomas have FCD around the tumor as determined by pathology.33 These tumors alone are well-known epileptogenic lesions; but pertinent to seizure control, FCD areas usually extend beyond the tumor. These areas of FCD in association with tumors may have epileptogenic mechanisms similar to FCD in nonlesional refractory epilepsy.32 When FCD is not resected, seizures can recur after surgery.29 This argument is supported by studies showing that more extensive resections attained a higher rate of seizure freedom.32
Thus, complete removal of the tumor is not sufficient to obtain seizure-free outcomes, and finding the area of FCD is important for total seizure control.32,34 In 1 study of patients with refractory epilepsy and gangliogliomas, 100% of patients without FCD remained seizure-free after surgery, whereas only 14% of patients with FCD remained seizure-free.33 However, MR imaging has a sensitivity as low as 5%–14% compared with pathology for defining the borders of FCD associated with glioneural tumors.32,33 Fortunately, the FCD associated with these tumors shows larger areas of hypometabolism. Thus, FDG-PET/MR imaging coregistration can be helpful in indentifying FCD when associated with tumors (Figs 4 and 5).
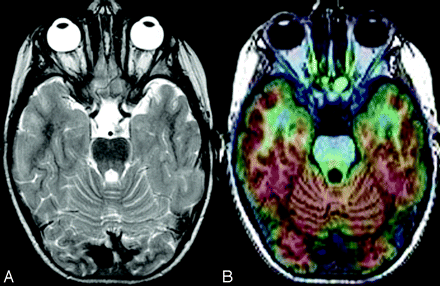
Tumor with surrounding FCD in a 7-year-old girl. A, T2-weighted MR image shows a lesion in the right mesiotemporal lobe with perilesional blurring of the gray and white matter in the right anterior temporal pole. B, FDG-PET/MR imaging coregistration demonstrates relative hypometabolism in right temporal lobe localized at the lesion and the surrounding abnormal gray and white matter. After a right anterior temporal lobectomy, pathologic findings of the surgical specimen showed a neoplasm with features of oligoastrocytoma with surrounding FCD type I.
Tumor with surrounding FCD in an 18-year-old woman. A, FLAIR sequence shows hyperintensity of the left amygdala and temporal lobe. B, FDG-PET/MR imaging coregistration demonstrates an area of hypometabolism extending beyond the lesion seen with MR imaging. After surgical resection, ganglioglioma was seen on pathology of the lesion (white arrow). Pathology of the area of hypometabolism surrounding the lesion (yellow arrow) showed histology consistent with FCD type I.
TSC
Tuberous sclerosis complex (TSC) is an autosomal dominant neurocutaneous syndrome with manifestations found throughout the body. In the brain, disordered proliferation, migration, and differentiation of neurons result in subependymal giant cell astrocytomas, subependymal nodules, and tubers. Starting in infancy, patients with TSC develop increasing seizure frequency and severity.35 In fact, 25%–50% of patients with TSC develop refractory epilepsy.35,36 Given the medically refractory nature of the epilepsy, surgery should be considered for such cases. The outcome of surgery is dependent on accurate presurgical assessment to guide resection of the epileptogenic tuber.37
However, presurgical evaluation has been challenging. Although CT and MR imaging can reveal the presence of multiple tubers, such anatomic imaging used alone cannot depict the epileptogenic tuber. Usually, multiple tubers are present, but seizures often arise from a single tuber.37 Additionally, noninvasive EEG techniques may not localize epileptogenic activity to a discrete tuber.37 Recently, a multimodality imaging approach by using FDG-PET/MR imaging coregistration and diffusion tensor imaging has been demonstrated to be useful in presurgical evaluation to localize the epileptogenic tubers.6 Larger volumes of FDG-PET interictal hypometabolism relative to tuber size on MR imaging and higher apparent diffusion coefficients in the subtuber white matter showed promise for detecting epileptogenic tubers and improving surgical outcomes (Fig 6). Thus a multimodality approach with FDG-PET/MR imaging coregistration should continue to be evaluated as a promising technique for noninvasive presurgical evaluation for TSC.
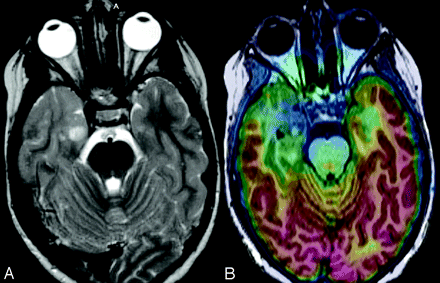
TSC in a 5-year-old girl. A, T2 axial image shows numerous hyperintense tubers on both temporal lobes. B, FDG-PET/MR imaging coregistration demonstrates an area of hypometabolism in the right temporal pole that is significantly larger than the tuber size. EEG demonstrated the presence of right temporal interictal discharges suggesting an epileptogenic zone in this area. Right temporal lobectomy was performed with good postsurgical seizure control.
Discussion and Future Directions
FDG-PET/MR imaging coregistration is a valuable tool for the presurgical evaluation of medically refractory epilepsy for the common underlying etiologies of MTS, FCD, epileptogenic tumors, and TSC. Our experience at UCLA has demonstrated this value.
Although numerous studies have already found MR imaging and FDG-PET to be complementary for presurgical evaluation of refractory epilepsy, there exists only a limited number of studies explicitly concerned with the use of coregistration of MR imaging and FDG-PET for this purpose.6–8,36 One retrospective study of FDG-PET/MR imaging coregistration used in consecutive surgeries for medically refractory epilepsy has already demonstrated a significant benefit in the presurgical evaluation, the surgery itself, and the postsurgical outcomes.8 Additionally, the benefits of FDG-PET/MR imaging coregistration for presurgical evaluation of patients with medically refractory epilepsy with TSC and FCD have already been demonstrated.6,7 In this review, we additionally describe the benefits of FDG-PET/MR imaging coregistration for MTS and epileptogenic tumors.
A potential challenge exists in that FDG-PET can sometimes falsely localize the epileptogenic activity.38,39 Hypometabolism is the typical finding on interictal FDG-PET.38,39 However, in ictal studies, FDG-PET may show hypermetabolism. Furthermore, ictal FDG-PET is often difficult to interpret because it often reveals complex patterns of both hypometabolism and hypermetabolism.39 Therefore, it is important to monitor the patient with scalp EEG continuously to verify if there is seizure activity observed at the time of 18F-FDG injection.
FDG-PET has proved to be a valuable addition to MR imaging for medically refractory epilepsy, but 18F-FDG is not the only PET tracer used for this purpose. 11C-labeled flumazenil and 11C-labeled-methyl-L-tryptophan have also been validated as PET tracers for epilepsy.40–42 These 2 tracers have been shown to be more useful compared with 18F-FDG for presurgical evaluation of certain conditions underlying epilepsy.40,41,43 Furthermore, numerous other PET tracers targeting different biochemical processes continue to be developed and evaluated for epilepsy.44,45 These innovative tracers may serve as powerful tools to facilitate presurgical evaluation of medically refractory epilepsy in the future.
Conclusions
The use of FDG-PET/MR imaging coregistration provides better structural and functional information noninvasively, which has been shown to improve the identification of epileptogenic foci, to aid surgical planning, and to lead to better postsurgical seizure control. We recommend the use of this technique for the presurgical evaluation of patients with medically refractory epilepsy, particularly in MR imaging with normal findings, and the continued effort to evaluate this technology for this patient population.
Indicates open access to non-subscribers at www.ajnr.org
References
- Copyright © American Society of Neuroradiology