Abstract
BACKGROUND AND PURPOSE: Our aim was to determine the effects of intra-arterial (IA) nicardipine infusion on the cerebral hemodynamics of patients with aneurysmal subarachnoid hemorrhage (aSAH)–induced vasospasm by using first-pass quantitative cine CT perfusion (CTP).
MATERIALS AND METHODS: Six patients post-aSAH with clinical and transcranial Doppler findings suggestive of vasospasm were evaluated by CT angiography and CTP immediately before angiography for possible vasospasm treatment. CTP was repeated immediately following IA nicardipine infusion. Maps of mean transit time (MTT), cerebral blood volume (CBV), and cerebral blood flow (CBF) were constructed and analyzed in a blinded manner. Corresponding regions of interest on these maps from the bilateral middle cerebral artery territories and, when appropriate, the bilateral anterior or posterior cerebral artery territories, were selected from the pre- and posttreatment scans. Normalized values were compared by repeated measures analysis of variance.
RESULTS: Angiographic vasospasm was confirmed in all patients. In 5 of the 6 patients, both CBF and MTT improved significantly in affected regions in response to nicardipine therapy (mean increase in CBF, 41 ± 43%; range, −9%–162%, P = .0004; mean decrease in MTT, 26 ± 24%; range, 0%–70%, P = .0002). In 1 patient, we were unable to quantify improvement in flow parameters due to section-selection differences between the pre- and posttreatment examinations.
CONCLUSIONS: IA nicardipine improves CBF and MTT in ischemic regions in patients with aSAH-induced vasospasm. Our data provide a tissue-level complement to the favorable effects of IA nicardipine reported on prior angiographic studies. CTP may provide a surrogate marker for monitoring the success of treatment strategies in patients with aSAH-induced vasospasm.
Delayed cerebral ischemia represents one of the leading causes of morbidity and mortality in patients with aneurysmal subarachnoid (aSAH) surviving the first 24 hours.1 Indeed, the presence of cerebral vasospasm has been associated with a 1.5 to threefold increase in mortality during the first 2 weeks after aSAH, and approximately 5%–10% of hospitalized patients with aSAH die from vasospasm.1,2 According to the Cooperative Aneurysm Study, 70% of the patients who present with aSAH develop vasospasm, with symptomatic brain ischemia or infarcts occurring in 36% of all patients.3 Thus, once the aneurysm is secured, one of the main focuses in the management of patients with aSAH is the prevention, detection, and treatment of vasospasm. The most commonly used approaches include oral nimodipine; induced hypertension, hemodilution, and hyperdynamic (triple-H) therapy; and endovascular techniques, including intra-arterial (IA) infusion of vasodilators, percutaneous transluminal balloon angioplasty (PTA), or a combination of the 2.
Given its short duration of action and several potential complications, papaverine has been largely replaced by other vasodilators such as milrinone,4 verapamil,5 nicardipine (Cardene IV),6 or nimodipine7 as the drug of choice for IA vasospasm treatment. Indeed, IA nicardipine infusion has become the primary endovascular vasospasm treatment at our hospital. Nicardipine is known to affect positively both angiographic (increasing vessel diameter) and clinical vasospasms.6 The degree to which nicardipine actually augments tissue-level perfusion, however, has not, to our knowledge, been quantified in humans. The value of CT perfusion (CTP) in the evaluation of patients with clinically significant aSAH-induced vasospasm has been previously demonstrated.8–11 The purpose of this study was, therefore, to determine the effects of IA nicardipine infusion on the cerebral hemodynamics of patients with aSAH-induced vasospasm as measured by first-pass quantitative cine CTP. In addition, we tested the hypothesis that CTP may be a useful tool in the quantification of the effects of vasospasm treatment. CTP may, therefore, provide a surrogate marker for comparing other potential treatments in future clinical trials.
Materials and Methods
Patients
The patients in this study were admitted to our hospital for the care of aSAH between September 2002 and June 2004. Approval was obtained from the institutional review board at our institution to conduct this retrospective review. Informed consent for endovascular treatment was obtained as part of routine clinical care. All patients with aSAH are treated under a standard protocol, which includes admission to the neuroscience intensive care unit for preoperative and postoperative monitoring. After surgical or endovascular treatment of the aneurysm, patients are routinely maintained in a relatively hypertensive and euvolemic state in anticipation of cerebral vasospasm. Daily clinical observations and transcranial Doppler (TCD) measurements are used to detect vasospasm. Patients with TCD findings suggestive of vasospasm are typically treated with induced hypertension (with phenylephrine and/or norepinephrine) and hypervolemia (crystalloids ± albumin to maintain central venous pressure above 12 mm Hg).
CT angiography (CTA) and concomitant first-pass quantitative cine CTP are typically performed in patients with clinical findings suggestive of vasospasm or TCD findings suggestive of vasospasm if a reliable neurologic assessment cannot be obtained (eg, intubated patients with poor baseline examinations). If vasospasm is confirmed by the CTA and/or CTP, then IA vasodilation with nicardipine and/or PTA are typically used. A retrospective review of our aSAH data base led to the identification of 6 patients who had CTP imaging just before and right after undergoing vasospasm treatment with IA nicardipine infusion, without any other treatment changes occurring between the CTP scans. These 6 patients are the subjects of this study.
Imaging Protocol
CT was performed by using a multidetector (16 sections) helical CT scanner (LightSpeed; GE Healthcare, Milwaukee, Wis) in the head-first supine orientation. An 18-gauge cannula was placed into an antecubital vein before the patient's entry into the scanner. Once in the scanner, the patient's head was immobilized and the contrast infusion pump was connected to the cannula. A precontrast axial study was obtained from the foramen magnum to the vertex. Immediately following this, a helical CTA scan was started 25 seconds after the beginning of a power infusion of 100 mL of nonionic contrast material at 4 mL/s (Omnipaque, 300 mg I/mL; Amersham Health, Princeton, NJ). The imaging parameters used during this phase were 140 kilovolt (peak) (kV[p]), 170 mA, 0.8-second rotation time, 2.5-mm section thickness, 512 × 512 image matrix, and standard algorithm reconstruction. Maximal attenuation projection and 3D images were then constructed by using GE Healthcare and Vitrea (Vital Images, Minnetonka, Minn) workstations.
First-pass CTP consisted of a cine series acquired after the administration of 50 mL of iodinated contrast material by using the same intravenous route at 7 mL/s with a 5-second delay. The imaging parameters used were 80 kV(p), 200 mA, 1-second rotation time, 4 contiguous sections (avoiding the lens whenever possible), 60 images per section, 5-mm section thickness, 512 × 512 image matrix, and standard algorithm reconstruction. Arterial input function (AIF) was selected from axial cuts of the supraclinoid internal carotid artery (ICA) or the A2 segment of the anterior cerebral artery (ACA).12 The same AIF was used for both pre- and posttreatment studies. CTP images were obtained just before and immediately after the endovascular vasospasm treatment, with <1 hour elapsing between each step.
Endovascular Treatment Protocol
Digital subtraction angiography (DSA) was performed by use of the transfemoral Seldinger technique. Once vasospasm was confirmed, nicardipine was diluted in 0.9% sodium chloride to a concentration of 0.1 mg/mL and administered in 1-mL aliquots through a Prowler microcatheter (Cordis Neurovascular, Miami Lakes, Fla) into the affected vascular territory (ICA terminus, M1 or A1 segments, or proximal basilar artery). All patients had a ventriculostomy catheter. If intracranial pressure (ICP) increased substantially (>20 mm Hg), the ventriculostomy was opened and allowed to drain to lower the ICP. Continuous blood pressure monitoring was also available in all patients through a radial arterial line. Cerebral perfusion pressure was maintained above 70 mm Hg throughout the procedure.
Data Processing and Analysis
The first-pass quantitative CTP data were transferred to a workstation (Advantage Windows; GE Healthcare) and analyzed with commercially available perfusion analysis software (CT Perfusion-2 software, FuncTool 3.0; GE Healthcare) to create maps of mean transit time (MTT), cerebral blood volume (CBV), and cerebral blood flow (CBF).
The CBF, CBV, and MTT data were transferred to a personal computer for analysis. Regions of interest were outlined by using a commercially available image-analysis program (ALICE; Hayden Image Processing Solutions, Denver, Colo) by an investigator blinded to the CTA and angiographic procedure results. One slab for each territory on each scan was selected on the basis of the levels that looked most comparable between the pre- and posttreatment scans. Region-of-interest contours were delineated in the bilateral middle cerebral artery (MCA) territories and, when appropriate, the bilateral anterior or posterior cerebral artery (ACA or PCA) territories on the pretreatment perfusion maps. The regions of interest were copied on the anatomically corresponding locations on the posttreatment CTP maps. For each region, mean pre- and posttreatment CBF, CBV, and MTT values were calculated.
Statistical Analysis
The relative rate of change (eg, percentage change in the posttreatment values when compared with the pretreatment values) in CBF, CBV, and MTT was individually calculated for each vascular territory. Comparison of pretreatment with posttreatment changes in CBF, CBV, and MTT was performed by using repeated measures analysis of variance with the patient as a fixed effect and pre- and posttreatment as the time component. A P value < .05 was considered significant. All computations were performed with the aid of SAS software (Version 9.1; SAS Institute, Cary, NC).
Results
The patient data including time of treatment in relation to the aSAH, age, sex, Hunt and Hess grade, Fisher group, location and treatment of the aneurysm, location and severity of the vasospasm, IA nicardipine dosage per location, and pre- and posttreatment CTP data are summarized in the Table.
Patient data summary
A total of 15 vessels were successfully treated in 6 patients. One patient underwent PTA in addition to IA nicardipine infusion. The remaining patients were treated only with IA nicardipine. The total dose ranged from 6 to 30 mg per patient. In 1 patient, we were unable to quantify improvement in flow parameters, due to section-selection differences between the pre- and posttreatment CTP examinations. This patient was not included in our quantitative analysis; however, subjective analysis of the 2 closest pre- and posttreatment sections was suggestive of posttreatment improvement.
A total of 16 vascular territories were analyzed in the remaining 5 patients, including bilateral MCA and ACA territories in 2 patients, bilateral MCA and PCA territories in 1 patient, and bilateral MCA territories in 2 patients. One patient (case 4) had near-complete occlusion of the M2 segment of the right MCA on angiographic evaluation. She was treated with 8 mg of nicardipine infusion in the right ICA, resulting in only minimal angiographic response. In another patient (case 5), only the left ICA was treated because the procedure had to be aborted due to seizures. In the remaining 14 vascular territories, treatment resulted in satisfactory angiographic response.
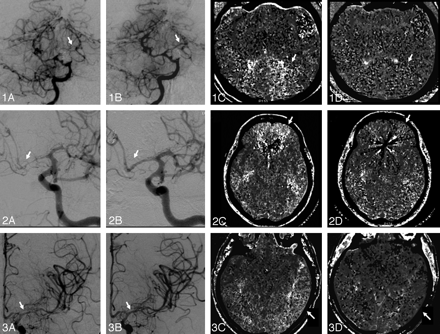
Both CBF and MTT improved significantly in the affected regions in response to IA nicardipine therapy. Mean CBF increased from 38.2 ± 13.1 mL/100 g/min in the pretreatment CTP scan to 50.7 ± 12.9 mL/100 g/min in the posttreatment CTP scan. This translates into a relative CBF increase of 41.3 ± 42.9% (range, −9%–162%; P = .0004). Mean MTT decreased from 6.0 ± 1.2 seconds in the pretreatment CTP scan to 4.3 ± 1.3 seconds in the posttreatment CTP scan, resulting in a relative MTT reduction of 26.2 ± 24.2% (range, 0%–70%; P = .0002). The CBV response was quite variable and did not show any specific pattern; mean CBV was 2.8 ± 0.7 mL/100 g in the pretreatment CTP scan and 2.9 ± 0.8 mL/100 g in the posttreatment CTP scan (relative CBV change, 4.0 ± 20.6%; range, −44%–34%; P = .63). Fig 1 illustrates the effects of IA nicardipine infusion on DSA and MTT maps.
Panels 1, 2, and 3 illustrate cases 1, 2, and 3, respectively. Panels A–D show pre- and posttreatment angiograms and MTT maps, respectively. Panel 1: A, Severe vasospasm of the left PCA, which had a marked response to nicardipine infusion in the basilar artery (1B) resulting in 28% reduction in MTT (1C, -D) and 62% increase in CBF (not shown) at the left PCA territory. Panel 2: A, Severe vasospasm of the left ACA, which had a marked response to PTA of the A1 segment and nicardipine infusion (2B), resulting in 68% reduction in MTT (2C, -D) and 89% increase in CBF (not shown) at the left ACA territory. Panel 3: A, Moderate vasospasm of the left MCA, which had only a mild angiographic response to nicardipine infusion (3B). C and D, Despite the suboptimal angiographic result, there was a 37% reduction in MTT and 69% increase in CBF (not shown) at the left MCA territory. This likely reflects the nicardipine effect in the microcirculation.
To assess the possibility that the aforementioned benefit was mostly related to PTA, we performed a second analysis excluding the 4 vascular territories (case 2) that were also treated with PTA. The improvement in cerebral hemodynamics remained statistically significant, with a relative CBF increase of 25.7 ± 25.9% (range, −9%–69%; P = .01) and a relative MTT reduction of 13.8 ± 10.5% (range, 0%–37%; P = .026).
Discussion
In the present study, we have described the direct effects of IA nicardipine on reduced CBF in patients with hemodynamically significant vasospasm after aSAH by using first-pass quantitative cine CTP. The principal findings of the study are: 1) the characterization of the acute CBF response to IA nicardipine infusion; and 2) the validation of the use of CTP in the assessment of new treatment modalities for vasospasm.
The mechanism by which aSAH-induced vasospasm occurs is not yet fully understood; however, both experimental and clinical data strongly suggest the efficacy of calcium antagonists in the treatment and prevention of vasospasm.13–15 Nimodipine, an oral dihydropyridine calcium antagonist, has been shown to reduce the incidence of vasospasm-induced cerebral infarction by 34% and the rate of poor outcomes by 40%.16 Nicardipine is another dihydropyridine calcium antagonist, which has more selective effects on vascular smooth muscle than on cardiac muscle. Several studies have demonstrated that continuous intravenous nicardipine infusion significantly decreases the incidence of symptomatic, angiographic, and TCD vasospasm.15,17,18 However, the efficacy of this regimen has been limited by prolonged hypotension, pulmonary edema, and renal dysfunction.
Badjatia et al6 have previously demonstrated that IA nicardipine infusion improves angiographic vasospasm with an effect more sustained than what has been reported with papaverine. However, it is clear that factors other than angiographic response play a role in the clinical response to vasospasm treatment as demonstrated by the lack of correlation between angiographic and clinical improvement in many situations. For instance, patients treated with IA papaverine may still have further neurologic deterioration despite angiographic improvement.19 Conversely, patients treated with IA nimodipine may demonstrate remarkable clinical improvement despite unchanged angiographic vasospasm.7 Similarly, IA infusion of L-arginine has been shown to markedly increase CBF in a primate model of SAH, despite the lack of any angiographic changes.20 Changes in perfusion at the microvasculature level probably account for this clinicoangiographic dissociation. Therefore, assessment of cerebral perfusion as opposed to angiographic results alone should be considered when evaluating novel therapies for vasospasm.
As an example, Firlik et al19 have demonstrated that IA papaverine may fail to improve CBF despite reversal of angiographic vasospasm. These authors reported a series of 15 patients with symptomatic vasospasm who underwent a total of 23 IA papaverine treatments (including combined angioplasty on 5 occasions). Angiographic vasospasm was at least partially reversed following treatment on 18 of 23 occasions (78%). Associated clinical improvement was major on 6 occasions, but either minor or none on 17. Pre- and posttreatment CBF was assessed by stable xenon-enhanced CT on 13 occasions but showed improvement in previously ischemic areas in only 6 of these (46%).19
CTP has been shown to be a useful tool in the selection of proper candidates for acute stroke thrombolysis21 and has more recently been used to diagnose and guide medical and endovascular therapy in patients with delayed cerebral ischemia after aSAH.8,9,22 The application of CTP to assess the response to vasospasm treatment has also been previously reported. Ono et al23 performed CTP in 8 patients with aSAH-induced vasospasm before, immediately after, and 4.5–6 hours after IA infusion of fasudil hydrochloride with an average CBF in the infused hemisphere of 41.6 ± 3.56, 46.4 ± 5.82, and 41.6 ± 7.42 mL/100 g/min, respectively. The authors concluded that, though initially effective, fasudil hydrochloride is a short-lived treatment for vasospasm.23 In our study, pretreatment and immediately posttreatment mean CBF was 38.2 ± 13.1 and 50.7 ± 12.9 mL/100 g/min, respectively.
Our analysis is somewhat limited by its overall small sample size and by the fact that PTA was performed in 1 of our patients, and differences in section selection did not allow the inclusion of that last patient in the analysis. In addition, our work carries all the limitations of a retrospective methodology. Finally, although we proved the effectiveness of IA nicardipine in increasing CBF in patients with vasospasm, we did not assess the duration of this effect due to concerns about the additional contrast and radiation exposure that would be necessary for another CTP examination. Despite these limitations, we were able to demonstrate a statistically significant improvement in CBF and MTT immediately after IA nicardipine infusion.
Conclusion
IA nicardipine infusion improves CBF and MTT in ischemic regions in patients with aSAH-induced vasospasm. Our data complement, at the tissue level, the previously reported favorable effects of IA nicardipine seen with angiographic studies. In addition, this study reinforces that CTA/CTP is a safe and promising technique for the evaluation of aSAH-induced vasospasm. Indeed, quantitative CTP may provide a surrogate marker for monitoring the success of treatment strategies in patients with aSAH-induced vasospasm. Future studies with larger sample sizes are required to confirm our results and to answer the questions about the duration of the treatment effect as well as its overall impact in clinical outcomes.
Footnotes
Paper previously presented in part at: Annual Meeting of the American Society of Neuroradiology, June 5–11, 2004; Seattle, Wash.
References
- Received March 28, 2008.
- Accepted after revision July 11, 2008.
- Copyright © American Society of Neuroradiology