Abstract
BACKGROUND AND PURPOSE: Visual field defects sometimes occur after temporal resection surgery. Our purpose was to evaluate the correlation between visual field defects caused by temporal lobe resection and the degree of resection of the Meyer loop, as assessed by diffusion tensor tractography.
MATERIALS AND METHODS: We examined 14 patients who underwent temporal resection for temporal lobe epilepsy. We obtained presurgical tractographies and then measured the distance between the temporal tip and the anterior limit of the Meyer loop (T-M distance). The degree of resection of the Meyer loop was defined as the distance from the anterior limit of the Meyer loop to the posterior limit of the temporal lobe resection (M-R distance). This was calculated by subtracting the T-M distance from the measured distance between the temporal tip and the posterior limit of the resection (T-R distance).
RESULTS: The mean T-M distance was 36.6 mm. The interindividual variation of the distance ranged from 30.0 to 43.2 mm. Although there was no statistically significant correlation between the extent of the visual field defect and the T-R distance, there was a statistically significant correlation between the degree of the visual field defect and the M-R distance.
CONCLUSION: The range of interindividual variation for the position of the Meyer loop was rather large, indicating that this variation is the key factor in visual field defects after temporal lobectomy, and the visual field defect appears to be predicted by presurgical tractography. Evaluation of the Meyer loop through the use of tractography seems to be a feasible method, which can be used to predict the visual field defect after temporal lobe resection.
Temporal resection, including anterior temporal lobectomy or selective amygdalohippocampectomy, is a widely accepted surgery for temporal lobe epilepsy.1,2 However, this method sometimes can cause optic tract injury in the temporal lobe (the Meyer loop), which may lead to a “pie in the sky”–shaped visual field defect after the surgery.3–5 The degree of the visual field defect that has been reported depends upon the anatomic range of the resection.3,6 In some of the patients with whom we have dealt, we found that almost the same extent of resection can result in different degrees of visual field defects. Our curiosity about the reason for these differences was the motivation to further study this phenomenon. We hypothesized that the variations noted for the extent of the postsurgical visual field defect are due to interindividual variations of the position of the Meyer loop within the temporal lobe.
Studies on diffusion tensor imaging have shown that tractography can be used to examine the optic radiation,7,8 and recently, there was a preliminary study about 2 cases of temporal lobe resection that were accessed by tractography of the optic tract.9 In the current study, we evaluated 14 patients who underwent temporal lobe resection surgery for temporal lobe epilepsy. In these patients, we used diffusion tensor tractography to delineate the Meyer loop and then evaluated the interindividual variation of its anterior limit. We also evaluated the correlation between the extent of the postsurgical visual field defect and the extent of the surgical resection both from the temporal tip and from the anterior limit of the Meyer loop. In addition, we also attempted to evaluate the correlation between the extent of the postsurgical visual field defect and the delineation of the Meyer loop observed during the postsurgical diffusion tensor tractography.
Methods
Subjects
We retrospectively examined imaging data for 14 patients with temporal lobe sclerosis who underwent temporal resection surgery for temporal lobe epilepsy. All patients were examined by using diffusion tensor MR imaging both pre- and postsurgery. There were 5 males and 9 females, ranging from 13 to 66 years of age, including 1 pediatric patient (13 years old). Methods used for the temporal lobe resection included anterior temporal lobectomy in 10 patients and selective amygdalohippocampectomy in 4. In 10 patients, there was a right laterality of the focus and surgery, whereas in 4 patients, there was left laterality. After the nature of the procedures had been fully explained, informed consent for the imaging study was obtained from all subjects or their families.
The patients were classified into 4 groups on the basis of the postsurgical visual field defect in the medial and lateral sectors of the upper quadrant visual field in the eye that underwent the surgical procedure. Postsurgical visual field defect was evaluated by using the Humphrey Visual Field Analyzer (Zeiss Humphrey Systems, Dublin, Calif). The groups were as follows (Fig 1): group A: no visual field defect in the upper quadrant visual field; group B, incomplete defect in the medial sector of the upper quadrant visual field; group C, complete defect in the medial sector and incomplete defect within the lateral sector; and group D, complete defect in both the medial and lateral sectors. Measurements of the visual fields were made within 3 months (20 days to 91 days; mean, 44.7 days) after the surgeries.
Schematic drawing of the classification by visual field defect. Fibers in the anterior part of the Meyer loop correspond to the medial sector, and posterior fibers correspond to the lateral sectors of the upper quadrant visual field on the contralateral side. Because the anterior part of the Meyer loop is more likely to be injured by a temporal lobe resection, visual defects due to temporal lobe resection begin at the medial sector in cases of slight injury, with severe cases spreading into the lateral sector. Group A, no visual field defect in the upper quadrant visual field. Group B, incomplete defect in the medial sector of the upper quadrant visual field. Group C, complete defect in the medial sector and incomplete defect within the lateral sector. Group D, complete defect in both medial and lateral sectors.
Imaging
A 1.5T clinical MR imaging unit (Magnetom Sonata; Siemens, Erlangen, Germany) was used to obtain the diffusion tensor images through the use of an echo-planar imaging (EPI) sequence (TR = 2300 ms, TE = 122 ms, b = 1000 s/mm2, 6-axis encoding, FOV = 230 mm, matrix = 128 × 128, section spacing = 3 mm, section thickness = 3 mm, 39 sections, averaging = 6). We also acquired regular structural T1-weighted (spin-echo, TR = 500, TE = 20) sagittal images both before and after surgery. Imaging studies were made within 1 month before and 3 months after the surgeries.
Diffusion tensors were computed and fiber tract maps were created by using a PC workstation with “dTV II” diffusion tensor imaging software, as developed by Masutani et al10,11 (University of Tokyo, available at: http://www.ut-radiology.umin.jp/people/masutani/dTV.htm). Interpolation along the z-axis was performed to obtain isotropic data. The eigenvector associated with the largest eigenvalue or the principal axis was assumed to represent the local fiber direction. The tracking algorithm moved along the principal axis. The diffusion tensor at the next location was determined from the adjacent voxels, with its principal axis then subsequently estimated. Tracking lines were traced in this way and were propagated until the fractional anisotropy fell below an assigned threshold of 0.18, though we did not set the angle threshold to track the steep turn of the Meyer loop.
Data Analysis
Presurgical tractographies of the Meyer loop (green in Fig 2) were obtained with the seed area in the white matter that was located anterior to the lateral geniculate body and with the target area located in the ipsilateral sagittal stratum on the coronal planes at the level of the trigone.12 We also tried to draw postsurgical tractographies of the Meyer loop. The seed and target areas for the presurgical tractographies were recorded, with identical seed and target areas used for postsurgical images.
Right temporal lobe epilepsy. Selective amygdalohippocampectomy was performed. A, The circle shows the seed area for the presurgical tractography. An identical seed area was used for drawing the postsurgical tractography. B, Tractography of the optic radiation (green) and uncinate fascicles is shown. Note that the 2 tracts are close together at the temporal stem. Thus, tractography of uncinate fascicles drawn in advance can be used as an assistant or a guide to draw the Meyer loop. C, The T-M distance is 41 mm for presurgical tractography (arrow). D, Postsurgical tractography of Meyer loop is also achieved in this patient.
To help recognize the most anterior point of the Meyer loop in the presurgical images, we obtained tractographies of the uncinate fasciculus (yellow in Fig 2), which is located just anterior to the most anterior point of the Meyer loop. The seed area in the white matter of the frontal lobe was located on the coronal planes at the tip of the frontal horn of the lateral ventricle, and the target area in white matter was located on the coronal planes at the tip of the inferior horn of the lateral ventricle in the ipsilateral temporal tip.12 We measured the distance between the anterior limit of the Meyer loop and the posterior limit of the uncinate fascicles to verify the credibility of the tractography. The measurements were performed independently by 2 operators who were blinded to the outcome of the surgery, and the reproducibility of the position of the Meyer loop was examined by calculating the correlation coefficient.
We measured the distance from the temporal tip to the anterior limit of the Meyer loop (T-M distance), which was delineated by diffusion tensor tractography that was superimposed on the sagittal image reconstructed from the b = 0 image of the diffusion tensor sequences. Because the b = 0 image using the EPI sequence could possibly be subjected to distortion from the susceptibility effect, a correction of the measured distance was performed through the use of the sagittal spin-echo image of a section identical to the reconstructed sagittal image. For the correction, we measured the distance from the temporal tip to the occipital pole on both the reconstructed image and the spin-echo sagittal image, with the ratio of the 2 distances then multiplied to measure the T-M distance. The mean value of the T-M distances measured by the 2 operators was used as the final value for evaluations.
On the postsurgical sagittal spin-echo image, the anatomic distance from the temporal tip to the posterior limit of the temporal lobe resection (T-R distance) was measured on the spin-echo image. To deal with potential errors of measurement in cases of irregular resection cavities, we performed the measurement at the superior wall of the inferior horn of the lateral ventricle, where the optic radiation was located. By using the previously mentioned calculations, we were able to determine the distance from the anterior limit of the Meyer loop and the posterior limit of the temporal lobe resection (M-R distance). The larger the value for the M-R distance, the larger was the extent of the resection of the optic tract by the temporal lobe resection. On the other hand, a minus value for the M-R distance indicated the space between the most anterior point of the Meyer loop and the most posterior point of the temporal lobe resection. We also performed the same measurements and calculations for the postsurgical tractographies.
Concerning the previously mentioned data, we examined the following points: correlation between the extent of the visual field defect and the T-R distance through the use of an analysis of variance (ANOVA) test, the interindividual variation of the T-M distance on presurgical tractography, the correlation between the extent of the visual field defect and the presurgical M-R distance through the use of an ANOVA test, and the traceability of the Meyer loop on the postsurgical diffusion tensor image.
Results
For the presurgical diffusion tensor images, on the side of the planned surgical procedure, tractography of the Meyer loop was obtained in all the cases (Figs 2,3). The distance between the anterior limit of the Meyer loop and the posterior limit of the uncinate fasciculus was 1.1 mm on average (ranging from 0 to 3.8 mm). In 7 cases, there was no gap between the Meyer loop and the uncinate fasciculus because both tracts seemed to be in contact with each other.
Right temporal lobe epilepsy. Anterior temporal lobectomy was performed. A, Presurgical tractography. Tractography of the Meyer loop is shown as the green tract. T-M distance is 35.6 mm. Uncinate fascicles are shown in the yellow tract. Note that there is no gap between the 2 tractographies. B, Postsurgical spin-echo sagittal image. An anterior temporal lobectomy has been performed. The T-R distance is 38 mm (arrow). Because the T-M distance is 35.6 mm on the presurgical tractography, the M-R distance is calculated to be +2.4 mm. C, For the postsurgical tractography, the Meyer loop could not be delineated; thus, only the dorsal optic radiation could be drawn. D, The visual field 2 months after the surgery. There is a complete visual field defect in the medial sector and a partial visual field defect in the lateral sector of the lateral upper quadrant visual field.
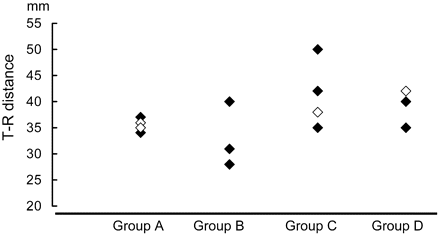
Figure 4 indicates the correlation between the extent of the visual field defect and the T-R distance. Although we performed an ANOVA test to examine the distance between the 4 visual field defect groups, there were no statistically significant differences shown for any of the total groups in any combination.
Correlation between the extent of the visual field defect and the T-R distance. Although there is a slight tendency for the T-R distance to appear larger in cases that have more severe visual field defects, overlap between the groups is quite large. No statistically significant differences could be observed by the ANOVA test. (Cases with anterior temporal lobectomy are shown in black, and cases with selective amygdalohippocampectomy are shown in white.)
The reproducibility of tractography by 2 operators is shown in Fig 5A. The correlation coefficient between the measurement of T-M distance by 2 operators was 0.97. The interindividual variation of the T-M distance is shown in Fig 5B. Although the mean distance was 36.6 mm, the distance ranged from 30.0 to 43.2 mm with an SD of 4.6 mm.
Interindividual variation of the T-M distance. A, The reproducibility of tractography by 2 operators. The correlation coefficient between the measurement of T-M distance by 2 operators is 0.97. B, Interindividual variations of mean T-M distances by 2 operators on a presurgical tractography. The mean T-M distance is 36.6 mm (SD = 4.45 mm). Variation ranges from 30.0 to 43.2 mm.
We found a correlation between the extent of the visual field defect and the M-R distance for the presurgical tractography (Fig 6). Mean M-R distance was −4.98 mm (SD = 1.73 mm) in group A, which indicated that for the presurgical tractography, there were still spaces as large as 4.98 mm on average between the most anterior point of the Meyer loop and the most posterior point of the temporal lobe resection. Similarly, the mean M-R distance was −5.46 mm (SD = 3.13 mm) in group B. On the other hand, the mean M-R distance was +5.97 mm (SD = 4.47 mm) in group C, which indicated that on average, 5.97 mm from the most anterior point of the Meyer loop had been resected during the surgery. Similarly, the mean M-R distance was +7.54 mm (SD = 2.26 mm) in group D. ANOVA showed statistically significant differences (P < .01) between groups A and C, groups A and D, groups B and C, and groups B and D.
Correlation between the extent of the visual field defect and the M-R distance. A plus value for the M-R distance indicates that there is a resected area of optic tract due to temporal lobe resection. A minus value for the M-R distance indicates that there are still spaces between the anterior limit of the Meyer loop and the posterior limit of the temporal lobe resection. The more severe the postsurgical visual field defect (groups C and D), the larger is the amount of resection of the Meyer loop. In contrast, minus values are seen for all the cases within groups A (no defect) and B (partial defect in the medial sector). ANOVA showed statistically significant differences (P < .01) between groups A and C, groups A and D, groups B and C, and groups B and D. (Cases with anterior temporal lobectomy are shown in black, and cases with selective amygdalohippocampectomy are shown in white.)
Using the identical seed and target areas of the presurgical tractographies, we attempted to draw postsurgical tractographies of the Meyer loop. However, postsurgical tractographies could only be obtained in 4 patients, including 3 patients in group A and 1 patient in group B. In all 4 of these patients, the presurgical M-R distances were found to have minus values.
Discussion
Temporal lobe epilepsy is one of the surgically treatable epilepsies, with operative methods that include anterior temporal lobectomy and selective amygdalohippocampectomy.1 Visual field defects occur frequently after temporal resections. This is due to the course of the optic radiations within the temporal lobe.13 The anterior part of the Meyer loop contains fibers corresponding to the medial part of the upper quadrant (medial sector, Fig 1), whereas in the posterior part of the Meyer loop, there are fibers that correspond to the lateral part of the upper quadrant (lateral sector, Fig 1). Because the anterior part of the Meyer loop is more likely to be injured during a temporal lobe resection, visual defects due to temporal lobe resection begin at the medial sector in minor cases and spread into the lateral sector in the more severe cases. When the injury to the Meyer loop becomes total, the patients develop complete upper quadrant visual field defects.14
There are several studies that have evaluated visual field defects after temporal lobe resections.3,6,15 One showed that there was a correlation between the extent of the resection and the visual field defect. Krolak-Salmon et al6 suggest that the anterior limit of the Meyer loop is located more rostrally than has been previously believed. However, in our study, no correlation could be proved between the visual field defect and the degree of resection from the temporal tips, as seen with conventional MR images. Although this may be due to resection lengths in the current study being narrower than those in the study by Krolak-Salmon et al, in which resection lengths were from 20 to 60 mm, we speculated that this was mainly due to the interindividual variation for the location of the Meyer loop. In the current study, though the mean T-M distance (which indicated the position of the Meyer loop) was 36.9 mm, the distance ranged from 29.9 to 43.3 mm, with SDs as large as 4.9 mm. Because there is a large T-M distance, uniform planning for temporal lobectomies may not be desirable with regard to preserving the optic tract.
The assessment on the correlation between the extent of the visual field defect and the presurgical M-R distance exhibited a positive result. In the current study, patients with severe postsurgical visual field defects (groups C and D) showed larger amounts of resection of the Meyer loop. In contrast, all the cases within groups A (no defects) and B (partial defects in the medial sector) exhibited minus values for the M-R distance, indicating there was space between the most anterior point of the Meyer loop and the most posterior point of the temporal lobe resection. Although no statistically significant differences could be proved between groups C and D, these results generally indicate that the degree of resection of the optic radiation itself and not the anatomic degree of resection in the temporal lobe affect the degree of the postsurgical visual field defect. Therefore, to predict the postsurgical visual field defect before surgery, the position of the Meyer loop needs to be evaluated in each individual patient by using diffusion tensor tractography.
Although we tried to evaluate postsurgical tractography of the optic radiation, in most cases, tractography could not be drawn. The reason for this was due to the loss of the optic radiation fibers themselves because without these fibers, tractography cannot be drawn even in cases where there is only mild or no visual field defect present. We theorize that edema or gliosis within the tissue adjacent to the resected area is responsible for the fractional anisotropy of the tissue, including the surgically preserved optic radiation, and it is this that leads to termination of the fractional anisotropy tracking.
In the current study, some limitations that might be present. The first has to do with the study population, because the number of patients and controls was rather small (n = 14). However, we were able to document a statistically significant positive correlation between the extent of the visual field defect and the presurgical M-R distance, whereas for the same reason, no significant correlation was obtained between T-R distances and postoperative visual field defects in this study. However, perhaps with a larger sample size, significant results may be obtained. The mean M-R value for group B was unexpectedly greater in absolute value than that of group A in the current study. This seems mainly due to small population size and 1 group B patient with a large M-R distance, with a larger amount of gliosis adjacent to the resection. Other patients also showed some extent of gliosis adjacent to the resections. It may be the reason that M-R distance is negative even in group B patients. In that sense, not only the degree of the resection but extent of gliosis may play some role on the degree of visual field defect. Another limitation may be the fact that this study was designed retrospectively. Because the operative method or degree of resection was based on clinical symptoms that were present, localization or extent of the epileptic focus, or the findings that had been determined by electroencephalography, the surgical plans did not take into consideration any tractography data that might have been available.
As has been shown in several studies, validation of diffusion tensor tractography is still a work in progress.16–18 During diffusion tensor tractography, the degree of visualization of the white matter tract tends to be less than the actual amount of the real white matter tracts that are present.17 To avoid the risk of undervisualizing the target tract (for example, the Meyer loop in this study), we drew a neighboring tract of the Meyer loop at the most anterior limit, which in this case was the uncinate fascicles. Kier et al19 reported that the posterior limit of the uncinate fascicles and the anterior limit of the Meyer loop meet closely, like kissing, within the temporal stem, by a postmortem MR imaging study with dissection.19 By using this knowledge, tractography of uncinate fascicles drawn in advance can be used as an assistant or a guide to draw the Meyer loop, indicating that the anterior limit of the Meyer loop will be close to the already-drawn posterior limit of uncinate fascicles. The gap between the posterior limit of the uncinate fascicles and the anterior limit of the Meyer loop was zero in 7 patients, and even in the 1 patient who had the largest gap, the length of this gap was no greater than 4 mm. Thus, by drawing the tractography of the uncinate fascicles, we were confident of the validity of the anterior limit that we determined during the tractography of the Meyer loop.
Lack of sophisticated distortion correction is also one of limitations in our study. Although we made a simple linear correction by multiplying the obtained distance by a ratio of the measured distance from the temporal tip to the occipital pole on the sagittal reconstructed b = 0 image to that obtained on a spin-echo sagittal image assumes that the EPI distortions are linear though the geometric distortions are nonlinear.
Our study population included patients who had undergone both anterior temporal lobectomy and selective amygdalohippocampectomy, with the data from all of these patients analyzed together. However, this combined analysis could also be considered to be a study limitation because there has been 1 study that found that visual field defects that occur after temporal lobe resection tend to be fewer in the patients having undergone selective amygdalohippocampectomy compared with those having undergone anterior temporal lobectomy.20 However, as shown in our figures, there was no tendency for the degree of the visual field defect to vary between the 2 operational methods that were used in this study population. This result fits with the findings of a larger study, which also found no significant difference in outcomes between the 2 operational methods.21 Thus, we consider that the degree of the optic tract injury at the Meyer loop may be the major factor that determines the degree of the visual field defect rather than the type of surgical method used.
Conclusion
Diffusion tensor tractography indicated that interindividual variation in the position of the Meyer loop can be quite large, which suggests that the extent of the temporal lobe resection seen with conventional imaging might not predict the postsurgical visual field defect. When using diffusion tensor tractography to evaluate the Meyer loop before surgery, we could predict postsurgical visual field defects more precisely. Although this is a preliminary study, which may be replicated by using larger sample sizes, it indicates potential application for MR tractography in clinical practice for identifying those patients at highest risk of postsurgical field defects by temporal lobe resection.
Acknowledgments
We thank Drs. Akio Fukusumi, Takeshi Wada, Kaoru Myochin, Toshiaki Akashi, Toshiteru Miyasaka, and Kentaro Tamura for their help in organizing this manuscript.
References
- Received December 27, 2007.
- Accepted after revision February 27, 2008.
- Copyright © American Society of Neuroradiology