Abstract
BACKGROUND AND PURPOSE: Diffusion-weighted imaging (DWI) can depict small punctate hyperintense lesions in the hippocampus in transient global amnesia (TGA). The purpose of this study was to find an optimal DWI protocol for lesion detection in TGA by investigating various imaging parameters and imaging timing after symptom onset.
MATERIALS AND METHODS: Sixteen patients with TGA diagnosed during 14 months underwent DWI within 24 hours and again at follow-up 3 days after onset. Each DWI session included 4 different sequences using different b-values (seconds per square millimeter) and section thicknesses (millimeter): 1000/5, 1000/3, 2000/3, and 3000/3. The presence or absence of hyperintense lesions on the 8 DWIs was determined visually, and the number of lesions detected was compared.
RESULTS: Thirteen of the 16 patients (81%) had either single or multiple punctate hyperintense lesions, totaling 24 lesions, and the remaining 3 patients had no lesions. All lesions detected were in the hippocampus except 1. The number of lesions detected on initial DWIs at a b-value/section thickness of 1000/5, 1000/3, 2000/3, and 3000/3 was 3, 9, 13, and 13, respectively, whereas that of follow-up DWIs was 17, 22, 24, and 24, respectively.
CONCLUSION: On the basis of these preliminary results, the highest lesion detection was achieved for DWI with b = 2000/3 mm or b = 3000/3 mm at 3 days postonset. When no lesion is detected by DWI within 24 hours after onset, follow-up DWI is recommended several days later.
Transient global amnesia (TGA) is clinically defined as sudden onset anterograde amnesia with preserved alertness, attention, and personal identity, which occurs during a period of no more than 24 hours with no long-term sequelae.1–3 The incidence of TGA has been reported to be 5–11 per 100,000 persons per annum.2,4,5 The etiology and pathogenesis of TGA are uncertain, though several different causes are suggested, such as ischemia, migraine, epileptic seizure, venous congestion, and psychological disturbances.6
Recent diffusion-weighted imaging (DWI) studies have indicated the presence of focal hyperintensities involving the hippocampus in TGA.7–11 The lesions detected by DWI are small and punctate (1–3 mm) and located within the lateral portion of the hippocampus.9,11,12 Since Strupp et al13 first detected hyperintense lesions in the hippocampus in TGA by using DWI, the frequency of lesions detected on DWI has been reported in a range of 0%–84%.9,10,14,15 According to a recent study, this discrepancy in detection rates appears to be attributable to the different timing of imaging from the onset of symptoms.9 In this previous study, a detection rate of only 6% by DWI was achieved within several hours of symptom onset, but this increased up to 84% at 48 hours post-symptom onset.
DWI parameters, such as b-value and section thickness, might also importantly influence the detection rate of the lesions. In particular, b-values higher than 1000 s/mm2 might increase the ability of DWI to detect subtle diffusion restrictions caused by small lesions. Moreover, section thicknesses of <5 mm may also increase the detection rate of small punctate lesions by decreasing partial volume averaging effects. However, optimal DWI parameters for lesion detection have not been studied previously. The purpose of the present study was to find an optimal DWI protocol for lesion detection by investigating b-values, section thickness, and imaging timing after symptom onset.
Materials and Methods
Patients
Twenty-two patients (18 women and 4 men; mean age, 60 ± 8 years; median, 61 years; age range, 46–74 years) with TGA, diagnosed by neurologists (J.S.L., S.Y.K.), were consecutively enrolled during the past 14 months. The criteria used to diagnose TGA were as follows1,16: 1) the presence of anterograde amnesia, 2) witnessed by an observer, 3) no clouding of consciousness or loss of personal identity, 4) cognitive impairment limited to amnesia, 5) no focal neurologic or epileptic signs, 6) no recent history of head trauma or seizures, and 7) resolution of symptoms within 24 hours.
Sixteen of the 22 patients underwent DWI twice by using our study protocol. The first DWI was performed within 24 hours, and the second, at 3 days after symptom onset. The other 6 patients were excluded because initial DWI was performed later than 24 hours after onset (2 patients) or no follow-up DWI was performed (4 patients). The ages of the 16 patients (14 women and 2 men) ranged from 49 to 69 years (mean, 59 ± 7 years; median, 60 years). Time to initial DWI after onset ranged from 3 hours to 22 hours 15 minutes (mean, 11 hours 5 minutes ± 7 hours; median, 9 hours), and time to follow-up DWI after onset ranged from 68 hours 45 minutes to 75 hours 30 minutes (mean, 72 hours 10 minutes ± 2 hours 40 minutes; median, 72 hours 30 minutes). This study was approved by the institution review board, and informed consent was obtained from patients or guardians.
MR Imaging
The MR imaging was performed on a 1.5T unit (Intera; Philips Medical Systems, Best, the Netherlands) with a sensitivity encoding (SENSE) head coil. Patients initially underwent MR imaging by using our routine stroke protocol plus the TGA protocol. The routine stroke protocol consisted of T1- and T2-weighted, fluid-attenuated inversion recovery, and conventional gradient-echo images in the transverse plane; T1-weighted images in the sagittal plane; 3D time-of-flight angiography of the intracranial region; and contrast-enhanced angiography of the neck region.
The TGA protocol consisted of 2 sessions of DWI (the first within 24 hours and the second at 3 days postonset). Initial DWIs were performed in the transverse plane covering the entire brain with 4 different sequences by varying b-values and section thickness as follows: 1) b = 1000 (s/mm2) and 5 mm, 2) b = 1000 and 3 mm, 3) b = 2000 and 3 mm, and 4) b = 3000 and 3 mm. Single-shot spin-echo echo-planar imaging was used with following parameters: matrix = 128 × 128 interpolated to 256 × 256, FOV = 220 mm, TR = 5000–12,500 ms (5000 ms at b = 1000, 9400 ms at b = 2000, 12,500 ms at b = 3000), TE = 60–75 ms, SENSE factor = 2, and number of acquisition = 4. Follow-up DWIs were performed by using these 4 different sequences in the transverse plane.
Imaging Analysis
DWIs of all patients were randomly ordered and analyzed. The presence or absence of hyperintense lesions on the 8 different DWIs was visually determined by consensus of 2 neuroradiologists (Y.C.W., J.H.K.). If a hyperintense lesion was detected on any of the 8 different DWIs, it was considered a positive lesion. If the lesions were within the hippocampus, specific locations (ie, unilateral versus bilateral, right versus left, and head versus body) were recorded. The number of lesions detected on each DWI was compared.
Results
Patient summary and lesion detection on 8 different DWIs are shown in the Table. Thirteen of the 16 patients (81%) had either single or multiple punctate (1–2 mm) hyperintense lesions, totaling 24 lesions. In the remaining 3 patients, no lesion was detected on any of the 8 DWIs. Seven of the 13 patients had a single lesion, 2 had 2 lesions, 3 had 3 lesions, and 1 had 4 lesions. With 1 lesion located in the right temporal lobe, 23 lesions were found in the hippocampus: unilateral in 7 patients and bilateral in 6 patients. Thirteen lesions were in the right hippocampus; 10 lesions, in the left hippocampus; 10 lesions, in the hippocampal head; and 13 lesions, in the body. No abnormal findings other than these lesions were detected on DWI and other MR images.
Patient summary and lesion detection on 8 different DWIs
The number of lesions detected on initial DWIs was 3 at b = 1000/5 mm, 9 at b = 1000/3 mm, 13 at b = 2000/3 mm, and 13 at b = 3000/3 mm, whereas that of follow-up DWIs was 17, 22, 24, and 24, respectively (Table). Assuming that follow-up DWIs at b = 2000 or 3000/3 mm depicted the lesions perfectly, we found that lesion-detection rates for the initial 4 DWIs were 13%, 38%, 54%, 54% in the same order as above and those of follow-up DWIs were 71%, 92%,100%, and 100%. Of the 24 lesions, 21 (88%) were not detected on initial DWI at b = 1000/5 mm (Fig 1). Seven lesions (29%) were still not detected on follow-up DWIs at b = 1000/5 mm but were detected at b = 1000/3 mm, b = 2000/3 mm, and b = 3000/3 mm (Fig 2).
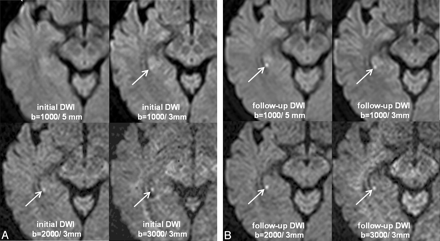
A 49-year-old woman (patient 8) with TGA. A, Initial DWIs obtained at 11 hours 15 minutes after the onset of symptoms show no lesion at b = 1000/5 mm, but a subtle bright lesion in the right hippocampal body (arrows) at b = 1000/3 mm, b = 2000/3 mm, and b = 3000/3 mm, with increasing lesion conspicuity by increasing the b-value. B, Follow-up DWIs after 3 days show a much brighter lesion (arrows) in all sequences.
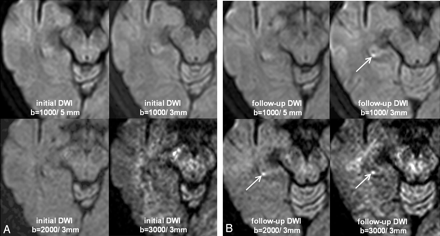
A 50-year-old woman (patient 3) with TGA. All initial DWIs (A) obtained at 4 hours 30 minutes after the onset of symptoms and follow-up DWIs (B) after 3 days at b = 1000/5 mm do not show the lesion. Follow-up DWIs at b = 1000/3 mm, b = 2000/3 mm, and b = 3000/3 mm show a bright lesion (arrows) in the right hippocampal body, though the signal intensity-to-noise ratio becomes poor by increasing the b-value.
Discussion
In the present study, DWI performed with a smaller section thickness (3 mm) was found to be better able to detect lesions than that performed at a 5-mm section thickness. Most of the previous DWI studies of TGA were conducted by using a section thickness of 5 mm and a b-value of 1000, except a recent study by Bartsch et al,11 in which a 3-mm section thickness was used. However, no comparison of lesion detection rates at different section thicknesses was conducted in their study. According to another recent study by Sedlaczek et al,9 using 5-mm DWI, the lesion was found in only 2 of 31 patients within 24 hours after onset. In the present study, the lesion detection rate within 24 hours after onset was 13% at 5-mm section thickness, but it increased up to 38% at 3 mm. Thus, in view of the small size of TGA lesions (1–3 mm), we think that a 5-mm section thickness is not optimal in detecting the lesions, particularly during the early stage. Moreover, these small lesions are more susceptible to partial volume averaging effect due to subtle diffusion restriction of the lesions at the early stage. Thus, DWI with a 3-mm section thickness is recommended for patients with suggested TGA within 24 hours after onset.
DWI obtained at higher b-values (b = 2000 or 3000) had higher lesion-detection rates than DWI at a b-value of 1000 in the present study. The lesion detection rate of the initial DWI was higher for b = 2000 or 3000 (54%) than for b = 1000 (38%) with equal section thicknesses (3 mm). As b-value increases, diffusion-weighting increases; therefore, higher b-values better enable the detection of the lesions with subtle diffusion restriction. However, the exponential loss of signal intensity for a given voxel with an increasing b-value is a trade-off for the increased diffusion-weighting at higher b-values,17 as shown in Figs 1 and 2. Moreover, no difference of lesion detection was found between b-values of 2000 and 3000. Thus, we recommend that a b-value of 2000 be used in patients with suggested TGA, particularly if DWI is performed within 24 hours after onset.
A higher detection rate was achieved on DWI performed at 3 days after onset compared with the initial DWI. This result concurred with the study by Sedlaczek et al,9 in which the lesion-detection rate was 6% within 8 hours of onset and increased up to 84% at 48 hours after onset. The pathogenesis of TGA is uncertain, and various etiologic hypotheses have been proposed, including thromboembolism,9,10 cerebral venous congestion induced by Valsalva-like activities,18–20 and vasoconstriction caused by hyperventilation.21 Whatever its etiology, several authors have suggested that delayed neuronal injury in the hippocampus is the cause of the delayed appearance of the lesions on DWI.9,22 It has also been speculated that delayed lesion appearance could be a result of progressive T2 prolongation of the lesions with time, as observed for thromboembolic infarctions.12 This speculation arises from the finding that small acute infarctions in the basal ganglia frequently have greater DWI signal intensities on the second or third days than on the first day post-symptom onset. Thus, although the pathogenesis of delayed lesion appearance is unclear, repeated DWIs at several days postonset are recommended, particularly when initial DWI findings are negative.
In the present study, multiple lesions were found within the hippocampus in almost half of the patients (46%). In recent published studies,9,11 which included >30 patients with TGA, multiple lesions were found in only 17, approximately 19% of the patients. We think that the higher incidence of multiple lesions in our study is due to the combined use of higher b- values and thin section thicknesses. The multiplicity of lesions may suggest an embolism as a conceivable etiology of TGA. The hippocampus is supplied by multiple hippocampal arteries, which are distal branches of the posterior cerebral artery.23 However, the findings that lesions developed exclusively in the hippocampus and that no lesions were found in other territories of the posterior cerebral artery do not support this embolic theory, though 1 lesion was found in the temporal lobe outside the hippocampus in the present study. Further discussion concerning the pathogenetic mechanism of TGA is beyond the scope of this study.
The presence or absence of the lesions on DWI in TGA may have no great impact on the treatment and prognosis of this benign self-resolving disease. However, there are several diseases, such as stroke, seizure, drug-induced amnesia, and other causes of altered awareness, that should be differentiated from TGA in the emergency department.24 Although the diagnostic criteria for TGA do not yet include DWI findings, an optimal DWI protocol can suggest the diagnosis even on the first day of the attack. Further imaging studies may provide novel insights on the pathogenetic mechanism of this mysterious disease.
There are some limitations in the present study, including a small number of patients, lack of control patients, and no gold standard (ie, no verification of a true lesion and a false lesion). Therefore, statistical analysis including sensitivity and specificity of lesion detection for each DWI protocol could not be performed. Instead, we found only an increasing trend of lesion detection by decreasing the section thickness, increasing the b-value, and repeating the imaging study after 2–3 days. Further refined studies are needed to overcome these drawbacks.
Conclusion
On the basis of our preliminary results, the combined use of a high b-value (b = 2000) and thin section thickness (3 mm) is preferred for the lesion detection of TGA. If no lesion is detected on initial DWI, especially if performed within several hours of symptom onset, follow-up DWI after several days is recommended.
References
- Received January 14, 2008.
- Accepted after revision February 24, 2008.
- Copyright © American Society of Neuroradiology