Abstract
BACKGROUND AND PURPOSE: Mamillary body and fornix asymmetry are frequent findings on MR imaging of the brain. We sought to determine the prevalence of asymmetry of the fornix and mamillary body on MR imaging in patients with or without seizures.
MATERIALS AND METHODS: MR images were retrospectively evaluated for asymmetry of the mamillary body and fornix in 178 patients who had a history of seizures, of whom 35 had suspected mesial temporal sclerosis (MTS). Additionally, 353 patients who had no limbic system pathology were reviewed. All patients were examined with spin-echo MR imaging, consisting of contiguous axial and/or coronal fluid-attenuated inversion recovery (FLAIR), T2-weighted, and sagittal T1-weighted imaging. Additionally, the patients with seizures had oblique coronal 3-mm T2-weighted, FLAIR, and 1.5-mm magnetization-preparation rapid gradient echo scanning through their temporal lobes.
RESULTS: In the patients who had no limbic system pathology or seizure history, 6.5% (23/353) had MR imaging evidence of asymmetric mamillary bodies and 7.9% (28/353) had asymmetric fornix size. Asymmetry of the mamillary body and fornix size was found in 37.1% (13/35) and 34.3% (12/35), respectively, of subjects with suggested hippocampal sclerosis. The prevalence of asymmetry of the mamillary body and fornix was statistically significantly higher in the patients with MTS (χ2 test, P <.0001).
CONCLUSION: Although asymmetry of the mamillary bodies and fornices is highly associated with MTS, this could also be seen as a normal variation or congenital abnormality.
In the examination for the source of seizures, many tools and methods have been used. The greatest consideration has been given to the study of the focal epilepsies. As a diagnostic or research tool for generalized epilepsy, radiologic imaging has not been extremely useful other than to exclude mass lesions. Temporal lobe epilepsy is a relatively well-defined epileptic syndrome with localized electroencephalographic abnormalities, memory dysfunction, and hippocampal atrophy or mesial temporal sclerosis (MTS) on MR imaging.1–3 MTS is the most common cause of temporal lobe epilepsy found at surgery. Several studies have confirmed that MR imaging is a reliable technique for locating the origin of temporal lobe epilepsy.4–9 It has been previously suggested that neuronal damage in the hippocampus may cause atrophy of the ipsilateral limbic system as a result of transneuronal degeneration.10
The purpose of this study was to determine the prevalence of asymmetry of the fornix and mamillary body on MR imaging in patients with or without seizures. These structures may be diminished in size in a greater proportion of patients with MTS.11–13 We hypothesized that asymmetry of the fornix and mamillary body is not only related to MTS but also could be present as a normal variation or other cause of epilepsy.
Materials and Methods
MR images were retrospectively evaluated for asymmetry of the mamillary body and fornix in 178 patients who had a history of seizures, of whom 35 had suggested MTS. Additionally, 353 patients who had no limbic system pathology on their images or clinical history were also reviewed. These patients were the groups of patients with seizure and the other patients without seizure history in a single institution (Johns Hopkins Hospital, Baltimore, Md). Subjects were selected on the basis of the clinical history provided to the neuroradiology service, which led to performance of a “seizure protocol” on the scanner.
All patients were examined with fast spin-echo MR imaging consisting of 5-mm contiguous axial and/or coronal fluid-attenuated inversion recovery (FLAIR), T2-weighted, and sagittal T1-weighted images. The patients who had no seizures also underwent axial and coronal postgadolinium T1-weighted imaging. Additionally, the patients with seizures underwent oblique coronal 3-mm T2-weighted, FLAIR, and 1.5-mm magnetization-prepared rapid gradient echo (MPRAGE) scanning through their temporal lobes.
In a blinded fashion, 1 observer (A.O.) visually evaluated the hippocampus, fornix, mamillary body, and ventricles for asymmetry. A randomly selected subset of scans (n = 20) was independently reviewed by the other observer (D.M.Y.) for interobserver agreement. The same subset of patients was also reviewed by the first observer (A.O.) for intrarater agreement.
Asymmetry of the fornix was evaluated on coronal MR images by comparing the cross-sectional areas of both fornices at the level of the rostral crus just posterior to the body of the fornix. Results of the fornix evaluation were classified into equal size (both fornices being equal in size) and asymmetrically small (1 fornix being smaller than the other) types. The size and position of the mamillary body were evaluated on T2-weighted axial, T1-weighted coronal, oblique coronal T2-weighted, and 1.5-mm MPRAGE scans. The mamillary bodies were also classified into equal size and asymmetrically small types. Ventricular asymmetry was evaluated visually and grouped into equal size and asymmetrically small (1 ventricle being asymmetrically smaller than the other one) types.
The frequency of asymmetry in the fornix and mamillary body was statistically compared between the patients with no limbic system abnormality and no seizure history and the patients with seizure history. Additionally presurgical patients with seizures and MTS and patients with seizures without MTS were compared. The frequency of obvious ventricular asymmetry was compared between the patients with and without seizure history and the patients with and without MTS. Also the relation between ventricular asymmetry and fornix asymmetry was evaluated.
The χ2 test was used to compare categoric data between the 2 groups. Interobserver and intraobserver variation was measured by using κ statistics. All statistical analyses were performed by using STATA, Version 8.0 (StataCorp, College Station, Tex).
Results
MR images of 178 patients (105 females, 73 males; mean age, 36.3 years; age range, 8–69 years) with a history of seizures and of 353 patients with no seizure history (219 females, 134 males; mean age, 49.2 years; age range, 7–87 years) were retrospectively evaluated. Thirty-five patients with clinically intractable temporal lobe epilepsy were thought to have MTS. Pathology was obtained in 19 (54.2%) patients who underwent unilateral temporal lobectomy. The presence of hippocampal sclerosis was shown in 14 (40%) patients by identification of gliosis and/or neuronal loss in Ammon horn. Not all patients with seizures had a history of temporal lobe epilepsy. Some had generalized seizures, absence seizures, or nontemporal focal seizures.
Asymmetry of the Mamillary Body and Fornix Size
In the patients who had no limbic system pathology or seizure history, 6.5% (23/353) had MR imaging evidence of asymmetric mamillary bodies and 7.9% (28/353) had asymmetric fornix size (Table 1 and Fig 1). There were no other abnormalities in the ipsilateral hemisphere in the patients without seizures and with small mamillary bodies. Seven patients had normal brain MR imaging findings, and 1 had contralateral frontal meningioma. Other findings were the following: Chiari malformations (n = 2); metastasis (n = 3; cerebellar, frontal, pontine lesions); primary brain tumors in the contralateral hemisphere including anaplastic astrocytoma in the brain stem, operated glioblastoma multiforme in the occipital region, frontal mixed glial tumor, central nervous system lymphoma (n = 4); orbital tumor (n = 1); small vessel ischemic changes (n = 3); hydrocephalus (n = 1); and a pineal lesion (n = 1).
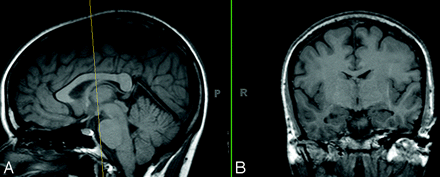
Asymmetric mamillary bodies. The coronal (B) T1-weighted images (TR/TE/TI, 600/15/1) demonstrate asymmetry of mamillary bodies in a patient with no seizure history.
Frequencies of asymmetry of mamillary bodies, fornices, and ventricles in each group (seizure and nonseizure)
Asymmetry of the mamillary body and fornix size was found in 14% (25/178) and 13.5% (24/178), respectively, of subjects with seizure history. In the patients with seizure without MTS, asymmetry of the mamillary body and fornix were found in 8.4% (12/143) and 8.4% (12/143), respectively (Table 2 and Fig 2). Asymmetry of the mamillary body and fornix size was found in 37.1% (13/35) and 34.3% (12/35), respectively, of subjects with MTS (Table 2 and Fig 3). Only 1 of the 35 subjects had a smaller mamillary body contralateral to the side of the MTS.
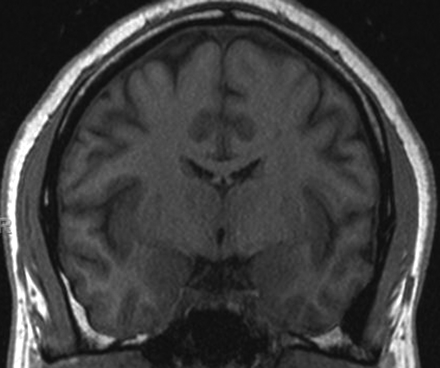
Left mamillary body volume loss. The coronal T1-weighted images (TR/TE/TI, 600/15/1) demonstrate a smaller mamillary body on the left side in a patient with seizures and no hippocampal sclerosis.
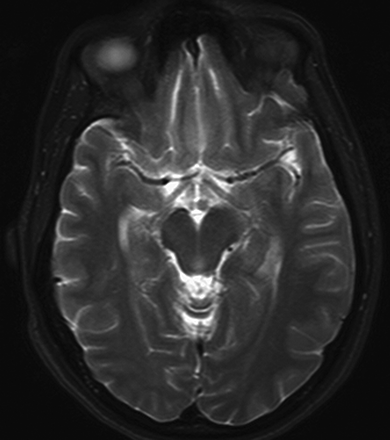
Mamillary body volume loss and MTS. The axial T2-weighted (TR/TE/TI, 4,000/98/1) image at the level of the mamillary bodies reveals a smaller mamillary body on the side of the hippocampal sclerosis.
Frequencies of asymmetry of mamillary bodies, fornices, and ventricles in the patients with seizures
Asymmetry of the Lateral Ventricles
The 353 patients without seizures had symmetric ventricles in 261 (73.9%), and 92 (26%) had ventricular asymmetry. In the seizure group, symmetric ventricles were present in 142 (79.8%) patients. According to these results, there was no significant difference in ventricle asymmetry between the seizure and nonseizure groups (Table 1) (P = .23). Four of the 6 patients with ventricular asymmetry in the MTS group also had fornix asymmetry (P = .005). Ten of the 30 patients with ventricular asymmetry in the group of patients with seizures without MTS also had fornix asymmetry (P = .001). Twenty-three patients with ventricular asymmetry (23/92) also had fornix asymmetry in the nonseizure group. Fornix asymmetry was significantly higher in the patients with ventricular asymmetry in both seizure and nonseizure groups (P = .001).
The frequency of an asymmetrically small mamillary body and fornix ipsilateral to the side of the smaller temporal lobe in the MTS group was significantly higher than that in the other groups (the patients without seizure history and the patients without MTS) (χ2 test; P < .001) (Fig 3). All except 1 patient with an asymmetrically small mamillary body in the MTS group also had an asymmetrically small fornix on the same side.
Inter-rater agreement for the subgroups of patients between the 2 observations was κ = 0.35 for the fornix, κ = 0.42 for the mamillary body, and κ = 0.26 for the ventricles. Intra-rater agreement in the same scans was κ = 0.60 for the fornix, κ = 0.86 for the mamillary body, and κ = 0.44 for the ventricles.
Discussion
Anatomy and Differentiation of Mamillary Bodies and Fornix
The limbic system constitutes the hippocampal formation and additional structures, such as the fornix, mamillary bodies, thalamus, cingulum, amygdala, and orbitofrontal cortex. The hippocampus is connected to the fornix, mamillary body, and anterior thalamic nucleus, forming part of the limbic system called the circuit of Papez.14–17 Anatomic differentiation of the fornix and mamillary bodies is relatively simple using MR imaging.
Abnormalities and Diseases of the Mamillary Bodies and Fornix
Although acquired diseases of the mamillary bodies have been well studied (eg, MTS, Wernicke encephalopathy), there are a few reported cases of congenital malformation of these structures.18–20 Congenital morphologic abnormalities of the mamillary body that could cause asymmetry in size, including hypoplasia or agenesis, have been rarely reported in autopsy cases, usually with other cerebral malformations.18
Possible Pathophysiologic Explanations of the Mamillary Body and Fornix Atrophy/Asymmetry in MTS
In MTS, cell loss and astrogliosis occur in the mesial temporal lobe, hippocampal formation, parahippocampal gyrus, amygdala, and entorhinal cortex. The proposed explanation for mamillary asymmetry in MTS is atrophy of the mamillary body caused by deafferentation related to injury of the medial temporal lobe. Neuronal loss in the subiculum causes fornical damage, and transneuronal damage can then occur in the mamillary body. The subicular damage is considered a major cause of the mamillary body atrophy and has been supported by several clinical reports.21,22 This effect is amplified after medial temporal surgery or stroke because there is a complete loss of input.12,13 Because the fibers that project to the mamillary body from the hippocampus course through the fornix, it is quite reasonable that asymmetry of the fornix may also be seen in some cases of MTS, especially with concomitant mamillary body volume loss.
MR Imaging Findings of MTS
The secondary findings in the mamillary bodies and fornix on MR imaging help in the diagnosis and lateralization of MTS. Primary findings include loss of the normal internal structure of hippocampus, temporal lobe or hippocampal volume loss, increased hippocampal signal intensity on T2-weighted/FLAIR scans, dilation of the temporal horn, narrowed collateral white matter, and blurring of the gray-white matter interface of the hippocampus.11–13,23,24 In patients with subtle primary findings of unilateral MTS, the secondary imaging features may help to improve diagnostic confidence.25 In cases of bilateral hippocampal abnormalities, secondary findings also may be helpful in determining the side more likely to account for the patient's symptoms. Although the secondary MR imaging findings associated with MTS are not sensitive predictors of this entity by themselves, they may offer clues in subtle cases.
MR Imaging Detection of Asymmetric Mamillary Bodies and Fornix
MR imaging detection of the asymmetrically small fornix11 or mamillary body26 has been suggested as a useful presurgical lateralizing sign of MTS in patients with temporal lobe epilepsy. Asymmetric mamillary bodies have been reported in an average of 20% of healthy subjects13,27 and 53% of presurgical patients with MTS.11,13,26 Additionally, asymmetric fornices have been reported, on average, in 33% of healthy subjects13,27 and 76% of patients with MTS.11,13,27 It is possible that “fornix atrophy” in previous studies has included normal variations because differentiation of fornix atrophy from physiologic asymmetry seems to be difficult.
Ventricular asymmetry frequently accompanies asymmetry of the fornices. Ventricular asymmetry is a common finding on MR images of the brain. An association between ventricular size and MTS might explain the high prevalence of thinning of the fornix reported by Baldwin et al.11 The difficulty would arise in differentiating such a case from the normally encountered ventricular asymmetry. Mamourian et al24 questioned whether fornix asymmetry is linked more to lateral ventricular size asymmetry than to temporal lobe epilepsy. The authors showed a statistically significant relation between lateral ventricular size and MTS, implying that an asymmetric fornix is due to the enlargement of the ventricle rather than to the sclerosis. Our study group also demonstrated a statistically significant relation between asymmetry of lateral ventricle and fornix. This relation suggests that asymmetry of fornix is due to enlargement of the ipsilateral ventricle.
There are some limitations to our retrospective visual inspection using conventional MR images to show asymmetry of the limbic system. Mamillary bodies and fornices are fairly small structures compared with the section thickness of 4–5 mm used in the patients without seizure in this study. If a section is tilted, the findings can easily be obscured by the partial volume effect. Future prospective studies including similar thin-section-thickness imaging in these patients will allow us to detect accurate prevalence and incidence of these structures. Partial volume effect in imaging might cause changes in the percentage of prevalence of mamillary body and fornical asymmetry. We also evaluated the ventricular asymmetry by visual inspection without quantifying the size volumetrically. However, the important question of this study was to determine prevalence of limbic system asymmetries in clinical studies with conventional MR imaging. Although quantitative techniques may increase sensitivity over visual inspection, in routine clinical practice, it is almost impossible to use such quantitative methods.25,28
Conclusion
Although asymmetry of the mamillary bodies and fornices is highly associated with MTS, this could also be seen as a normal variation or congenital abnormality. From a clinical viewpoint, extrahippocampal structural changes associated with MTS may not alter seizure outcome after surgery29; however, reporting MR imaging findings of asymmetric mamillary bodies and fornices can help in the detection, index of suspicion, and lateralization of MTS.
References
- Received May 23, 2007.
- Accepted after revision July 22, 2007.
- Copyright © American Society of Neuroradiology