Abstract
BACKGROUND AND PURPOSE: Arterial reocclusion and distal embolization are known complications of ischemic stroke intervention, impacting treatment strategies and device design. We sought to determine their rates of occurrence and effects on long-term outcomes during endovascular treatment of patients with acute ischemic stroke.
MATERIALS AND METHODS: Retrospective analysis of data from 4 prospective acute stroke protocols was performed. Patients underwent the standard technique for parent vessel angiography followed by pharmacologic thrombolysis and/or sonographic thrombolysis and/or mechanical thrombus disruption. Certain patients also received systemic heparin or abciximab therapy. Demographic, clinical, and angiographic variables were assessed at onset, 24 hours, 1 week, and 1–3 months after the event. “Distal embolization” was defined qualitatively as appearance of an occlusion on a downstream vessel. “Arterial reocclusion” was defined as subsequent reocclusion of the target vessel after initial recanalization had been achieved.
RESULTS: Arterial reocclusion occurred in 18% of these patients, whereas distal embolization occurred in 16% of the 91 patients treated in these protocols. Arterial reocclusion, but not distal embolization, was associated with a lower likelihood of favorable outcome at 1–3 months (P = .05; odds ratio, 3.9; 95% confidence interval, 0.01–0.98) after adjusting for age, initial National Institutes of Health Stroke Scale scores, sex, time to treatment, initial angiographic grade, symptomatic intracranial hemorrhage, and final recanalization.
CONCLUSIONS: Arterial reocclusion and distal embolization occur in 16%–18% of patients with stroke undergoing endovascular intervention. Only arterial reocclusion is associated with poor long-term outcome. Prospective studies are needed to identify risk factors for their occurrence and possible preventive therapies.
Although it is generally believed that vessel recanalization leads to improved outcome in acute ischemic stroke,1–3 complications of thrombolysis, such as arterial reocclusion (Fig 1) and downstream embolization of thrombus (Fig 2), may counter the benefits of therapy. Early reocclusion following thrombolysis has been shown by transcranial Doppler (TCD) to occur in 34% of patients receiving intravenous (IV) recombinant tissue plasminogen activator (rtPA) and may result in neurologic worsening in many of these patients.4 Preliminary studies of arterial reocclusion during endovascular treatment have shown rates of 17%, with a subsequent association with poor outcome.5 The concern for arterial reocclusion may lead physicians to administer anticoagulation or platelet glycoprotein (GP) IIb/IIIa inhibitors in patients undergoing endovascular therapy,6,7 despite the increased risk of intracranial hemorrhage.8
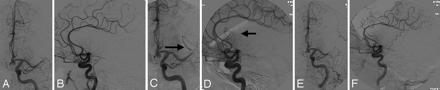
Arterial reocclusion. A 54-year-old woman, status post IV tissue plasminogen activator, has a left M1 occlusion seen in the anteroposterior (A) and lateral (B) planes (Qureshi grade 3A). After mechanical thrombolysis, there is improved flow in the superior division, frontal branches of M2 (arrows, C and D; Qureshi grade 2). However, the final angiogram shows reocclusion of the M1 stem similar to her initial angiographic appearance (E and F, Thrombolysis in Myocardial Infarction grade 0, Qureshi grade 3A).
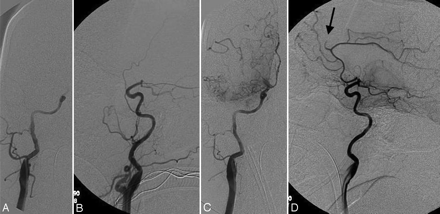
Distal embolization. A 53-year-old woman has right carotid terminus occlusion seen in the anteroposterior (panel A) and lateral (panel B) projections (Qureshi grade 5). Status post IA reteplase and intravenous abciximab, there is flow through the anterior cerebral artery seen in the anteroposterior (C) and lateral (D) projections. The arrow (D) indicates an occlusion of the callosomarginal branch, whereas the pericallosal branch is filling well (Qureshi grade 3B).
Concern for distal embolization has impacted device design in ischemic stroke therapy, serving as the rationale for the use of distal protection devices in carotid artery stent-placement procedures9–11 and the basis of the balloon-guide catheter for the Merci retriever (Concentric Medical, Mountain View, Calif), though exact rates of distal embolization were not known in the MERCI trial.12,13 Studies of distal embolization during carotid artery stent placement procedures, by using diffusion-weighted imaging (DWI) techniques as a marker, estimate occurrence rates of 17%–30%, even with the use of distal protection devices.14–16
We sought to evaluate the occurrence of distal embolization and arterial reocclusion in patients undergoing revascularization therapy in 4 prospective acute stroke protocols: intra-arterial (IA) reteplase and mechanical thrombus disruption (MTD, group 1),5,17,18 the EKOS MicroLysUS North American trial (group 2),19 MTD following IV thrombolysis (group 3),20 and IA reteplase and IV abciximab (FDA IND 9180, group 4).21
Materials and Methods
Patient Selection and Thrombolysis Procedure
Patients older than 18 years of age (and ≤77 years for the EKOS trial19) presenting with signs and symptoms of acute ischemic stroke and consenting to treatment in 1 of 4 prospective trials were included. All studies were performed under the approval of institutional review board at the respective sites. All patients underwent digital subtraction angiography (DSA) with a guiding catheter placed in the carotid or vertebral artery.
IA Reteplase and MTD
Patients with a National Institutes of Health Stroke Scale (NIHSS) score22 >16 or those beyond 3 hours or with other exclusionary criteria to IV rtPA23 were offered local IA thrombolysis with reteplase (maximal dose, 8 U).5,17,18 After initial DSA, a RapidTransit or Prowler Plus microcatheter (Cordis, Miami Lakes, Fla) was used to access the target vessel for local IA thrombolysis.5,17,18 For refractory thrombi, a 2- to 4-mm Amplatz gooseneck snare (Microvena, White Bear Lake, Minn) or a balloon-tipped microcatheter was used to perform MTD.5,17,18
EKOS MicroLysUS North American Trial
All patients enrolled in the North American safety and feasibility study to evaluate the MicroLysUS infusion catheter (EKOS, Bothell, Wash) were included.19 Inclusion and exclusion criteria for this trial have been described previously. In general, patients had an NIHSS score ≥8 and were within 6 hours of onset for suspected anterior circulation or 24 hours for a suspected posterior circulation ischemic event.19
DSA followed protocol similar to that described previously for parent vessel angiography. The 2.5F MicroLysUS 2.1-MHz sonographic-tipped infusion catheter was advanced through the guiding catheter to the target vessel. Sonographic transmission at a power of 0.21–0.45 W was begun, followed by an IA bolus of either 2 mg of rtPA or 0.4 U of reteplase and a subsequent infusion of 0.3 mg/min (maximal dose of 20–30 mg) for 60–120 minutes or 8 U of reteplase for 60 minutes. Angiograms were obtained at 15-minute intervals. IV heparin (2000 U) was administered after the identification of an arterial occlusion, followed by a 500-U/h infusion for 4 hours.19
MTD Following IV Thrombolysis
Patients presenting within 3 hours of acute ischemic stroke onset and with an initial NIHSS score of ≥10 underwent emergent cerebral angiography and mechanical angioplasty or snare manipulation procedures following administration of IV rtPA (0.9 mg/kg).
IA Reteplase and IV Abciximab
For patients presenting between 3 and 6 hours of acute ischemic stroke, urgent angiography and thrombolysis, as described previously, were performed. After demonstration of an arterial occlusion by DSA, patients received IV abciximab (0.25-mg/kg bolus followed by 0.125-μg/kg per minute infusion for 12 hours) and IA reteplase, reconstituted as 2 units in 20 mL of normal saline in escalating doses (0.5–2 U). DSA was performed after the instillation of each 0.25-U aliquot of reteplase.21
Clinical Evaluation and Angiographic Review
Demographic and baseline data, including the number of patients per study protocol, age, sex, and occlusion site, were collected for all patients. Patients were assigned an NIHSS score on the basis of their clinical examination at presentation and 24 hours and 7 days after the event. Modified Rankin Scale (mRS) scores24 were assessed at 1–3 months after the event. CT scans were obtained 24–48 hours after treatment to assess the presence of intracranial hemorrhage.
Angiograms were graded according to the Qureshi angiographic grading scheme25 on the initial DSA films demonstrating the flow through the target vessel. The Qureshi grading scheme classifies occlusions as 0 (no occlusion), 1 (1 branch vessel occlusion), 2 (2 branch vessel occlusions), 3A or 3B (M1 stem occlusion with preserved or lost lenticulostriate vessels), 4A or 4B (internal carotid artery or basilar artery occlusion with collaterally supplied antegrade or retrograde flow), and 5 (complete internal carotid artery or basilar artery occlusion). All subsequent angiographic images were reviewed for the presence of distal embolization, defined as a newly apparent occlusion in a downstream vessel after a revascularization maneuver, and for the presence of arterial reocclusion after initial partial or complete recanalization in the target vessel.5 The final angiographic grade was categorized as no, partial, or complete recanalization of the target occlusion, measured as a decrease in the final grade to a score >0 (partial recanalization) or a decrease in the final grade to 0 (complete recanalization).
Statistical Analysis
Descriptive statistics were used to calculate means and SDs for age, mRS, angiographic score, time to treat, proportion of subgroups of patients in each study, initial occlusion site, mean NIHSS score at all time points, and rates of distal embolization and arterial reocclusion. Student t tests and χ2 tests were used to compare demographic, clinical, and angiographic variables for both arterial reocclusion and distal embolization subgroups and unaffected patients. A multiple logistic regression analysis was used to assess the effect of arterial reocclusion and distal embolization on long-term outcome, controlling for age, initial NIHSS score, sex, time to treatment, dichotomized initial Qureshi grades (grade 0–3B and 4A-5), symptomatic intracranial hemorrhage, and final recanalization. Statistical analysis was performed by using the Statistical Package for the Social Sciences, Version 11.0 (SPSS, Chicago, Ill).
Results
Patient Recruitment per Study Protocol
A total of 100 patients were recruited in all 4 trials, of whom 9 were excluded due to lack of availability of angiograms for this review (n = 3), missing long-term outcome data (n = 1), or no occlusion at initial angiography (n = 5), leaving 91 patients. Their grouping according to study protocol was as follows: IA reteplase and MTD (n = 47, 52%); EKOS MicroLysUS North American trial (n = 12, 13%); MTD and IV thrombolysis (n = 19, 21%); IA reteplase and IV abciximab (n = 13, 14%). Distal embolization occurred in 15 of 91 patients, and arterial reocclusion of the target vessel, in 16 of 91 patients. Most arterial reocclusions were seen among patients in group 1, the largest cohort (n = 9, 56%), with the breakdown among other study protocols as follows: group 2, n = 4 (25%); group 3, n = 3 (19%); and group 4, n = 0 (P = .18, Table 1). In comparison, most cases of distal embolization were seen in group 2, n = 6 (40%), followed by group 3, n = 4 (27%); group 4, n = 3 (20%); and group 1, n = 2 (13%, P = .00, Table 2). Rates of no, partial, and complete recanalization according to treatment protocol were as follows: 13%, 57%, and 39% in group 1; 10%, 40%, 50% in group 2 (however data were incompletely recorded for the final analysis for 2 of these patients and thus not included in calculation of overall percentages); 32%, 42%, and 26%, respectively, in group 3; and 46%, 15%, and 39% in group 4 (n = 13). Results are shown in Table 3. Taken as an aggregate, complete recanalization was achieved in 32% of the patients in this analysis.
Comparison of patients with and without arterial reocclusion
Comparison of patients with and without distal embolization
Overall recanalization rates per treatment protocol
Demographic, Clinical, and Angiographic Data
Nearly half of the patients were men (n = 48, 8 in the arterial reocclusion and 6 in the distal embolization subgroups); the mean age was 67 ± 15 years. Patients having arterial reocclusions were on average older (70 ± 14 years) than the overall group (67 ± 15 years), whereas patients with distal embolization were younger (60 ± 13 years). Middle cerebral artery sites of occlusion predominated among all patients, (total, n = 48, 53%; arterial reocclusion, n = 9, 56%; distal embolization, n = 6, 40%), followed by internal carotid artery, of which 1 occurred in the cervical internal carotid artery (total, n = 29, 32%; arterial reocclusion, n = 5, 31%; distal embolization, n = 6, 40%), and basilar artery locations (total, n = 12, 13%; arterial reocclusion, n = 2, 13%; distal embolization, n = 3, 20%; Tables 1 and 2).
The median NIHSS score at initial presentation was 19 ± 8 for the overall group, compared with 19 ± 7 for the group with arterial reocclusion and 19 ± 8 for the distal embolization subgroup. Median NIHSS score at 24 hours was 18 ± 13 for the entire cohort, compared with 22 ± 11 for the arterial reocclusion subgroup and 24 ± 15 for the group with distal embolization. One-week median NIHSS scores were 18 ± 16 for the overall group, compared with 25 ± 16 for the arterial reocclusion subgroup and 21 ± 17 for the distal embolization subgroup. None of these differences between subgroups and the overall group were significant (Tables 1 and 2). Mean time to treat was shortest among the subgroup experiencing arterial reocclusion (4.05 ± 1.27 hours), compared with times >4.5 hours for all other patients (P = .06, Table 1). The mean angiographic Qureshi grades at onset and procedure termination were 3B and 2, respectively for all groups. General groupings of grades between 0 and 3B and 4A-5 at onset and procedure termination are presented for each group in Tables 1 and 2.
Outcome Data
Only 1 patient with arterial reocclusion (6%) had a favorable mRS at 1–3 months, defined as a score of 2 or better, compared with 28 (37%) patients without arterial reocclusion (P = .01).
After patients who never experienced recanalization (treatment nonresponders) were excluded from the analysis, similar low rates of favorable outcome (6% versus 41%) were seen among the subgroup who experienced arterial reocclusion (P = .01, Table 1). Rates of favorable mRS at 1–3 months were also lower among the subgroup with distal embolization compared with those without it (n = 2, 13% versus n = 27, 36%; P = .09), though this difference was only significant when excluding treatment nonresponders. In a stepwise logistic regression model, arterial reocclusion along with failed recanalization and severity of initial NIHSS score were independently associated with poor clinical outcome (mRS of ≤3) at 1–3 months (P = .05; odds ratio, 3.9; 95% confidence interval, 0.01–0.98) after adjusting for age (continuous variable), sex (dichotomous), time to treatment (continuous), initial angiographic severity of occlusion (dichotomized as grades 0–3B versus grades 4A-5), and the presence or absence of symptomatic intracranial hemorrhage. Distal embolization was not independently associated with poor clinical outcome. Arterial reocclusion and distal embolization occurred simultaneously in 6 patients but were not associated with an additive risk of poor mRS (Table 4).
Independent predictors of poor outcome (mRS ≥3)
Discussion
This retrospective analysis of patients with acute ischemic stroke undergoing multimodal endovascular interventions demonstrates arterial reocclusion rates of 18% and distal embolization rates of 16%. Despite the limitations of a retrospective analysis, the combined endovascular and systemic therapies studied here are valuable in their applicability to a generalized group of revascularized patients with acute ischemic stroke.
Arterial reocclusion, but not distal embolization, strongly predicted poor mRS at 1–3 months, recapitulating the fact that recanalization is important for improved outcomes in ischemic stroke.1–3 In contrast, the failure of distal embolization to predict poor long-term outcomes may have important implications in terms of recanalization therapy, where traditionally, caution regarding potential consequences of downstream thrombus embolization can limit efforts to recanalize the vessel.
Although effort was made to maintain objective criteria in analyzing the angiograms for the appearance of arterial reocclusion and distal embolization, analysis of distal embolization was confounded in certain circumstances, such as carotid terminus occlusions, in which it might be difficult to interpret the meaning of a downstream occlusion after an intervention, when the initial proximal occlusion precluded filling of the distal vessel. Also, the above reported rate of distal embolization occurring in only 6 of the 29 patients with internal carotid artery occlusions may seem lower than rates reported in the literature, but it is again confounded by the fact that 8 of these 29 patients had no or minimal revascularization.
Although key observations were made as to the type of treatment and occurrence of arterial reocclusion or distal embolization, the retrospective nonrandomized nature of this study limits us in drawing firm conclusions. Higher frequency of distal embolization occurred among the EKOS cohort, but this did not subsequently impact outcome. Although it seems intuitive that mechanical maneuvers may have higher rates of distal embolization, 20% in our analysis occurred in patients receiving solely pharmacologic therapy (IA reteplase and IV abciximab). In this analysis, the highest rates of complete recanalization were also seen in these groups: EKOS group (50%) and the IA reteplase/IV abciximab group (39%), suggesting that distal embolization may be a by-product of procedure success.
Studies of rates of distal embolization during acute revascularization procedures in the setting of acute carotid occlusion are limited. Imai et al26 report on 14 patients with differing methods of revascularization therapy, of whom 10 also had proximal flow arrest by means of a balloon-guide catheter. In their retrospective series, only a single instance of distal embolization occurred. Prospective studies would be needed to identify whether specific endovascular therapies are associated with successful recanalization and prevention of arterial reocclusion at the expense of distal embolization, and if arterial reocclusion ultimately impacts outcome.
Fewer instances of arterial reocclusion (n = 3) occurred in patients receiving systemic thrombolysis (group 3) and abciximab (group 4), compared with 15 of the patients in the remaining 2 groups. Again, the benefit of concomitant systemic therapy in suitable candidates to prevent the complication of arterial reocclusion may offer a fruitful area of future research. The breakdown of patients with arterial reocclusion by study protocol largely follows the rank order of the number of patients in each trial. Although no cases of arterial reocclusion were seen in the IA reteplase and IV abciximab groups, the overall lower numbers of this cohort preclude deriving any conclusion as to the benefit of GP IIb/IIIa inhibitors in preventing arterial reocclusion. From this analysis, we can only say that GP IIb/IIIa inhibitors may offer a useful area of study for acute ischemic stroke treatment. Assessment of postprocedural follow-up by angiography or other noninvasive imaging (eg, MR angiography, etc) would also be of interest in such an analysis.
The subgroup of patients experiencing arterial reocclusion had a trend toward shorter treatment times, as seen in similar reports of arterial reocclusion following IV rtPA documented by continuous TCD monitoring, in which patients who recanalized early had higher rates of reocclusion.4 This may represent a patient population that is in a dynamic reactive vascular state, prone to repeated thromboses and occlusions, a hypothesis that would help explain the association of poor outcome with arterial reocclusion, independent of recanalization state, because arterial reocclusion may be a marker of this vulnerable period, extending from the vascular status to the clinical state.
Perhaps the most compelling result from this analysis is the clear association of arterial reocclusion with poor outcome. It is estimated, on the basis of data from TCD,4 that more than one third of patients who receive IV rtPA may experience arterial reocclusion, which may lead to poor outcomes among those patients. Patients who receive IV rtPA as the sole therapy may not undergo early imaging of their vascular anatomy as would patients receiving endovascular therapies; thus, potentially treatable arterial reocclusion would be missed. Although we cannot state on the basis of these data that all patients receiving IV thrombolysis should have immediate vascular imaging, our analysis supports data shown by others that arterial reocclusion is of grave clinical consequence.4
Conclusion
Arterial reocclusion among patients with acute ischemic stroke receiving endovascular intervention occurs at a rate of 18% and is an independent predictor of poor long-term outcome. Distal embolization occurs in 16% of these patients but does not appear to impact outcome. Prospective studies are needed to identify systemic and endovascular treatment therapies that may impact the arterial reocclusion and distal embolization and their subsequent effects on outcome.
Footnotes
Centocor (Horsham, Pa) provided grant support and the pharmacologic agent for some of the patients enrolled in the IA reteplase and IV abciximab (FDA IND 9180) protocol, though Centocor did not review the results of this analysis. The EKOS Corporation (Bothell, Wash) provided angiograms and clinical data for the authors’ independent review for the purposes of this analysis. They also provided the EKOS MicroLysUS infusion catheters and endovascular sonography devices for the patients enrolled in the feasibility analysis of their device, whose data were pooled for analysis in this study. The EKOS Corporation did not analyze the data for this analysis.
References
- Received April 14, 2007.
- Accepted after revision August 4, 2007.
- Copyright © American Society of Neuroradiology