Abstract
BACKGROUND AND PURPOSE: Giant cell granuloma (GCG) is a rare lesion. The purpose of this study was to determine the CT characteristics and describe possible MR imaging features of GCG of the craniofacial bones.
METHODS: We retrospectively reviewed 7 CT studies and 1 MR imaging study of 7 histologically proved cases of GCG in 2 men, 3 women, and 2 patients of unknown gender, aged 12–51 years, during a period of 10 years, from 1995 to 2005.
RESULTS: The granulomas predominantly involved the maxilla in 3 patients, the mandible in 2 patients, the temporal bone in 1 patient, and the nasal cavity in 1 patient. These lesions on imaging were expansile masses that demonstrated adjacent bone wall thinning, and most were associated with lytic bone destruction. They were predominantly masses with soft-tissue attenuation on CT scans and may have infiltrated the surrounding soft-tissue structures. The patient with an MR imaging had a lesion that was hypointense on both T1- and T2-weighted MR images. The lesions revealed avid homogeneous contrast enhancement.
CONCLUSION: The imaging features of GCG are nonspecific. However, this entity should be included in the differential diagnosis of expansile lesions in the craniofacial bones.
Jaffe1 first coined the term “giant cell reparative granuloma” in 1953, when he described the lesion as a local reparative reaction to intraosseous hemorrhage induced by trauma. Before this time, this lesion was thought to be a giant cell tumor (GCT) or a GCT variant. There has been much debate concerning the pathogenesis of this lesion because it has been reported without a definite history of antecedent trauma and with a lack of significant elements of reparative tissue.2 This debate has led some to omit the adjective “reparative” from the name of the lesion because there is a question as to whether this truly represents a reparative process, especially on the basis of the pathologic findings; therefore, it is now referred to as a giant cell granuloma (GCG). Other theories of pathogenesis have been proposed, including infectious and developmental causes, but there has been no consensus to date about the etiology of the lesion.3, 4 Although incidence and prevalence rates for GCG have not yet been accurately determined, it is an uncommon benign non-neoplastic lesion that most often occurs in the bones of the mandible and maxilla. Gnathic cases of GCG are classified according to location as central (those occurring in bone) or peripheral (those occurring in gingival soft tissues) and have been reported as 1%–7% of all benign lesions.5–7 The most common extragnathic sites are the skull base and the small bones of the hands and feet.8–12
Patients typically present with localized disease that has an insidious natural clinical course, with solitary lesions identified incidentally. Despite the characteristic histologic and immunohistochemical features, GCG remains a diagnostic challenge to both clinicians and radiologists. To our knowledge, there are no studies larger than individual case reports that address the radiographic features of GCG; thus, knowledge of the imaging features of this entity in the literature is limited. In this study, we sought to describe the CT and MR imaging appearances of GCG in the mandible, maxilla, and skull base in 7 patients.
Methods
The clinical data and imaging studies of 7 patients with histopathologically confirmed GCG involving the mandible, maxilla, and skull base, during 10 years, from 1995 to 2005, were retrospectively reviewed from an institutional digital teaching file. Certain demographic and clinical information was not available for 2 of the 7 patients. This study included 2 men, 3 women, and 2 patients of unknown gender, aged 12–51 years (mean age, 31.8 years). Six patients underwent contrast-enhanced CT examination, and 1 patient underwent both contrast-enhanced CT and MR imaging examinations. The 4 CT scans were obtained at our institution by using a spiral CT scanner with 2.5-mm collimation and a 2.5-mm interval from the orbital roof through the face, with the injection of the contrast agent (iohexol, Omnipaque 300, GE Healthcare Medical Diagnostics, Buckinghamshire, UK). The images were obtained with soft-tissue and bone algorithms and in the respective window settings. The outside CT studies had similar imaging parameters. MR imaging was performed by using a 1.5T MR imaging unit (Signa, GE Medical Systems, Milwaukee, Wis) equipped with a head coil. One patient underwent an axial T1-weighted spin-echo sequence (TR/TE, 578/12; number of excitations, 2; percent phase field of view, 87.5; section thickness, 5 mm with no gap; matrix size, 256 × 256) and an axial T2-weighted fast spin-echo sequence (TR/TE, 5253/93; number of excitations, 2; echo train length, 16; percent phase field of view, 87.5; section thickness, 5 mm with no gap; matrix size, 256 × 256). In addition, contrast-enhanced T1-weighted spin-echo images were obtained in the axial and coronal planes after a bolus injection of 0.1 mmol/kg of gadolinium dimeglumine.
Images were reviewed by consensus by 2 certificate of added qualification board-certified neuroradiologists (R.H.W., H.R.H.) with knowledge of only the histologic diagnosis. Both CT and MR images were evaluated for the location and extent of the lesion, bone remodeling or destruction, and soft-tissue characteristics. Attempted follow-up times ranged from 0 to 10 years.
Histologic evaluation was performed on the biopsy specimens obtained from the tumors. The diagnosis of GCG was established on the basis of the presence of fibroblast-like spindle cells as well as focal groups of multinucleated giant cells surrounding a focus of hemorrhage and/or hemosiderin deposition.
Results
Presenting symptoms were pain (n = 2), facial mass (n = 2), and proptosis (n = 1). Two lesions were found incidentally at routine dental examination. One patient had a history of left temporal and forehead pain 20 years previous to the present presentation, which was treated with radio-frequency lesioning of the second branch of the trigeminal nerve. All patients underwent surgical resection or curettage of the lesion. Recurrence has been reported in 1 case.
Lesion Location and Extent
Of the 3 patients with lesions located primarily in the maxilla, 1 patient had a lesion located on the posterolateral right maxillary sinus wall, also involving the posterior maxillary ridge and extending to the left posterior second molar. The second patient had complete occlusion of the right maxillary sinus with midline deviation of the nasal septum secondary to mass effect. In the third patient, the lesion was seen on the left alveolar ridge of the maxilla, elevating the floor of the left maxillary sinus (Fig 1). One patient with a lesion not clearly centered within the bone had a GCG arising from the pterygopalatine fossa and extending along the vidian nerve, with expansion of the anterior vidian canal (Fig 2). Another patient had a lesion centered in the right nasal cavity, which extended from the inferior orbital wall to the hard palate and right maxillary alveolar ridge craniocaudally and crossed midline (Fig 3). Two patients with lesions involving the mandible had the typical expansile changes associated with GCG, both of which crossed midline.
Case 1. Images in a 51-year-old man with a history of painful maxillary swelling caused by GCG.
A, Contrast-enhanced axial CT image demonstrates an expansile lesion arising from the anterior and inferior left maxilla, with heterogeneous enhancement and soft tissue seen anterior to the maxilla.
B, Bone algorithm–correlating image demonstrates the osseous expansile changes, with thinning of the anterior wall and central foci of increased attenuation consistent with mineralization seen with GCG.
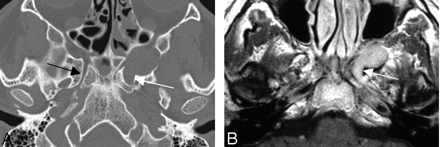
Case 2. Images in a 38-year-old man with a 20-year history of intermittent left-temporal and forehead pain caused by a GCG arising from the left pterygopalatine fossa.
A, Axial CT bone algorithm image demonstrates a lesion arising from the left pterygopalatine fossa. There are surrounding osseous expansile changes including expansion of the anterior left vidian canal (white arrow) compared with the normal right vidian canal (black arrow).
B, Correlating contrast-enhanced axial T1-weighted image shows avid homogeneous enhancement of this lesion, again with the expansion of the anterior left vidian canal (white arrow).
Case 3. Images in a 39-year-old woman with a history of a slowly expanding mass in the nasal cavity caused by a GCG of the right nasal vault.
A, Axial postcontrast CT image shows an avid homogeneously enhancing lesion arising from the anterior right nasal vault, with thinning and expansion of the surrounding osseous anatomic landscape extending into the right maxillary sinus.
B, Coronal postcontrast CT image also shows the osseous expansile changes of the surrounding anatomic landscape, with extreme thinning and secondary obstruction of the paranasal sinus air cells.
Bone Expansion and Remodeling
In all 7 patients, there were osseous expansile changes with thinning of surrounding cortical bone. Bone remodeling was evident in 6 of the cases, with osseous erosions noted at the margins of the lesions. Scattered foci of mineralization were seen on all except 1 case, which gave these lesions a multiloculated appearance. One lesion (Fig 2) was not centered within the bone, but instead within the pterygopalatine fossa, remodeling the surrounding osseous anatomic landscape.
Soft-Tissue Characteristics
Contrast-enhanced CT imaging of these lesions demonstrated soft-tissue attenuation expansile lesions, with surrounding mass effect. Postcontrast images revealed avid homogeneous enhancement.
Discussion
GCG are rare non-neoplastic lesions that are histologically distinct from GCTs of a bone.13 They account for a small percentage of all head and neck masses. The incidence is highest in the second decade of life with a female-to-male ratio of 2:1.14 There have been reports of accelerated growth and recurrence during pregnancy and in the postpartum period, which suggests that GCG may be hormone-dependent.7 No racial predilection is noted. Most recorded lesions are located in the mandibular or maxillary regions, with rare lesions occurring in the other craniofacial bones and small tubular bones of the hands and feet.3, 9, 11, 12 Manifestations in rare sites, including the axial skeleton and long bones, have been reported.8, 10 GCG most often appears as a slowly growing mass; therefore, a delay in diagnosis is common. The clinical presentation is mainly due to mass effect and depends on the site of involvement. The 2 most common clinical findings are soft-tissue swelling and pain. Proptosis, nasal obstruction, and cranial nerve palsy are less common nonspecific findings in the initial presentation.
To the best of our knowledge, there are no case series larger than individual case reports that address the CT and MR imaging findings of GCG. In this study, all 7 GCGs were bulky and appeared as heterogeneous soft-tissue attenuating masses with avid homogeneous contrast enhancement on CT scans. CT bone algorithm demonstrated that 6 of these lesions showed expansion with remodeling of the adjacent bone and lytic bone destruction as well as intralesional mineralization. These appeared to be multilocular. One lesion, though an expansile soft-tissue attenuating mass, lacked CT features common to the other 6, including lytic bone destruction and multilocularity. This lesion was seen invading and expanding the vidian canal, which was demonstrated on both CT and MR imaging. MR imaging offers advantages over CT because of its high soft-tissue contrast and multiplanar depictions. Postgadolinium MR imaging of this lesion demonstrated marked homogeneous enhancement. These imaging features are not specific, and bone remodeling and lytic bone destruction, though suggestive of the diagnosis, could be caused by other aggressive lesions.
Imaging alone cannot be used to reliably distinguish this lesion from other tumors and lesions of the craniofacial bones, such as giant cell tumor, aneurysmal bone cyst, ameloblastoma, and brown tumor of hyperparathyroidism. Together, CT and MR imaging are the methods of choice for assessing local involvement, but the diagnosis of GCG of the craniofacial bones still requires histologic and immunohistochemical evidence. Macroscopically, GCG has variable appearances. This lesion is histopathologically characterized by a connective tissue stroma composed of oval and spindle-shaped fibroblastic cells with multiple areas of hemorrhage, hemosiderin pigment, and abundant fibrosis. The multinucleated giant cells of GCG are strongly immunoreactive for CD68, suggesting histiocyte or macrophage origin. GCTs share similar histologic and immunohistochemical characteristics of GCG, including the presence of multinucleated giant cells and CD68 reactivity.15 It is important to distinguish between these 2 lesions because GCTs carry a higher incidence of recurrence, metastasis, and malignant transformation.16
Surgical resection continues to be the mainstay of treatment for GCG.4 Recurrence rates, in general, are low, ranging from 10%–15%.3, 4 The use of radiation therapy for the elimination of residual tumor remains controversial. Findlay et al17 reported that GCGs are not sensitive to radiation therapy, and they discouraged its use because of sarcomatous transformation. However, more recent reports suggest that such transformations have been reduced in incidence now that megavoltage rather than orthovoltage radiation is the standard mode of therapy.16
The study of GCG has potential limitations. The rarity of the lesion may have prevented the demonstration of more variations in the imaging features of this disorder, especially for MR imaging, because only 1 study was available. In addition, the radiologists were not blinded to the patient’s clinical history. This bias might have influenced the described CT and/or MR imaging findings. Because of the retrospective nature of this study, further studies are required to investigate correlations between the extent of the lesion, the imaging characteristics, and, ultimately, the clinical outcome.
Conclusion
GCG shows nonspecific CT and MR imaging features. However, features that may suggest the diagnosis are an intraosseous expansile soft-tissue mass that may infiltrate the surrounding soft tissues. Foci of mineralization are often present, and the lesions are generally multilocular. The lesion does not usually become disseminated, but it may be locally aggressive and demonstrate marked involvement and destruction of the adjacent bony structures. CT and MR imaging are complementary techniques in evaluating the local extent of this reactive process. GCG should be included in the differential diagnosis of expansile craniofacial masses because it has imaging findings similar to those of other more common lesions such as ameloblastoma, aneurysmal bone cyst, and brown tumor of hyperparathyroidism and because it can mimic a GCT, which carries a worse prognosis.
References
- Received October 16, 2005.
- Accepted after revision December 10, 2005.
- Copyright © American Society of Neuroradiology