Abstract
Summary: We report on a patient with oligodendroglioma metastatic to bone, presenting with pancytopenia and fever 10 years after initial tumor resection. Our review of the literature showed a total of 30 reported extraneural metastases, with only 19 of these being similar cases of bone metastases. These bony lesions have increased signal intensity in T2-weighted and low signal intensity on T1-weighted images, with intense homogeneous enhancement. However, on MR imaging, we were unable to find necrosis or compression deformity of the vertebrae, despite extensive metastatic disease.
Oligodendroglioma is a diffuse glial tumor. Extraneural metastasis of a primary intracranial neoplasm is rare in general and usually occurs only in the setting of prior neurosurgical resection. Metastasis of oligodendroglioma outside of the central nervous system occurs even more infrequently. There are multiple reports of oligodendroglioma spreading along cerebrospinal fluid pathways and only rare reports of metastases elsewhere. We report a case of oligodendroglioma metastasizing to the bone following 3 resections, 10 years after initial diagnosis. The patient presented clinically with complete bone marrow failure due to replacement of the bone marrow cells by tumor cells. A brief discussion of possible reasons for the rarity of extraneural metastases is presented along with a short review of the literature.
Case Report
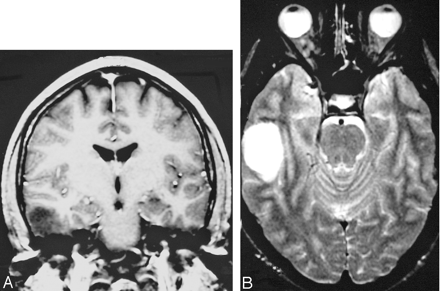
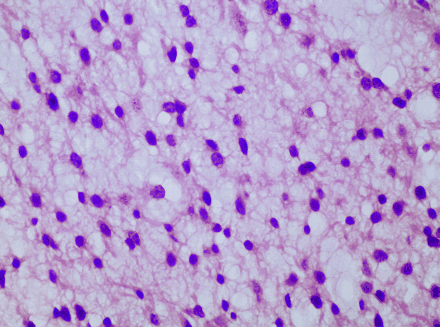
A 28-year-old man initially presented with a headache. MR imaging of the brain demonstrated a 2 × 2.5 × 2 cm mass in the right temporal lobe in the regions of the middle and inferior temporal gyri (Fig 1A, -B). He underwent resection of the tumor with pathology showing a hypercellular lesion composed of a uniform population of small glial elements with round-to-oval nuclei and relatively scant cytoplasm (Fig 2). Some perinuclear halos and neuronal satellitosis were noted along with areas of a background of delicate vascular capillaries. Cytoplasm did not stain positive for glial fibrillary acidic protein. The patient was diagnosed with low-grade oligodendroglioma.
The figures show a small well-delineated nonenhancing right temporal lobe mass with predominately low signal intensity in T1-weighted (A) and predominately increased signal intensity in T2-weighted (B) images, involving white and gray marrow consistent with oligodendroglioma.
The neoplasm is of moderate cellularity and composed of relatively uniform cells with round nuclei. Mitotic figures or pleomorphism is not identified.
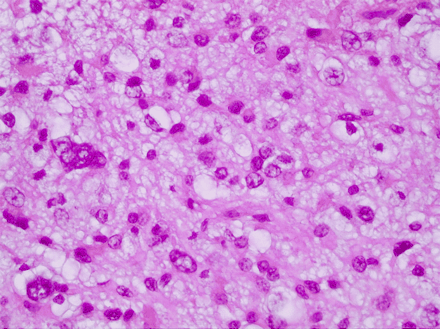
A repeat resection was performed 4 years later (Fig 3A, -B). Pathology showed low-grade oligodendroglioma with similar histology. Four years later, the tumor again progressed (Fig 4A–C) and was partially resected, followed by postoperative radiation of 6000 cGy. Pathology at this time showed high-grade (anaplastic) oligodendroglioma (Fig 5). Following this treatment, the patient was placed on chemotherapy (temozolomide, Temodar, Schering Corporation, Kenilworth, NJ), but this was discontinued after only 3 doses because of uncontrolled nausea and vomiting.
Four years later, interval tumor recurrence is seen in the posterior aspect of the surgical cavity with a homogeneous increased signal intensity seen in the T2-weighted (A) image and low signal intensity in the T1-weighted (B) image without enhancement.
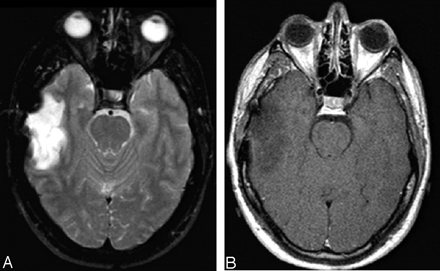
A second tumor recurrence is seen 8 years after initial diagnosis. A large homogenous mass is present, abutting the right lateral ventricle with low signal intensity on T1-weighted (A) and increased signal intensity on T2-weighted (B) images. No evidence of abnormal enhancement and of either subependymal or leptomeningeal spread is seen on the coronal T1-weighted postgadolinium (C) image.
Histologic image shows anaplastic oligodendroglioma with increased cellularity, with mitotic activity and increased nuclear pleomorphism.
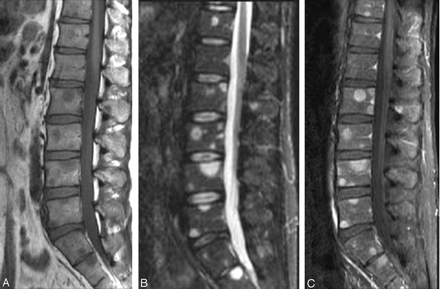
A month later the patient was hospitalized for back pain. MR imaging of the spine showed diffuse patchy increased signal intensity of the vertebral bodies and posterior elements on the T2-weighted images, consistent with metastases (Fig 6A–C). A biopsy of the T7 posterior spinous process showed bone marrow necrosis but no tumor cells. Findings of extensive metastatic evaluation to look for a new primary malignancy were also negative.
There are numerous areas of signal-intensity abnormality throughout the vertebral body with abnormal low signal intensity in T1-weighted (A) images corresponding to increased signal intensity on T2-weighted (B) images and diffuse abnormal enhancement after gadolinium administration (C). Note the absence of a compression deformity despite the presence of diffuse bony metastases.
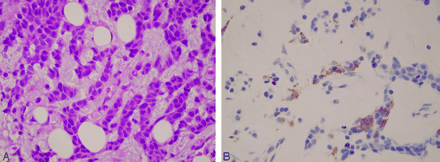
Four months after the T7 biopsy, the patient was readmitted to the hospital with fever and pancytopenia. A bone marrow biopsy of the iliac crest showed replacement of the patient’s marrow by malignant cells (Fig 7A, -B), which exhibited nuclear pleomorphism but were not histologically identical to his anaplastic oligodendroglioma. However, the diagnosis of metastatic oligodendroglioma to the bone marrow was based on the immunohistochemical staining. The tumor cells identified in the bone marrow spaces as well as in the primary anaplastic oligodendroglioma in the brain were positive for the glial fibrillary acidic protein marker, which is positive in glial, schwannian, and ependymal tumors (all neural tumors). Also, these cells were positive for the S100 marker; this finding added validity to the central nervous system origin of the metastatic cells. Furthermore, findings were negative in other pertinent immunohistochemical stains: cytokeratin, as would be expected in a carcinoma; lymphoid marker, as would be expected in lymphoma; and the endothelial marker, which is typical for vascular tumor. Multiple outside pathologists confirmed this diagnosis. The fact that a few months before, at the time of the diagnosis of bony metastases, findings of an extensive search were negative for another primary malignancy added validity to the immunohistostaining findings.
Metastatic poorly differentiated neoplasm in the bone marrow with scant cytoplasm and large hyperchromatic nuclei (A). The same specimen shows cytoplasmic staining for glial fibrillary acidic protein in tumor cells in the bone marrow (B).
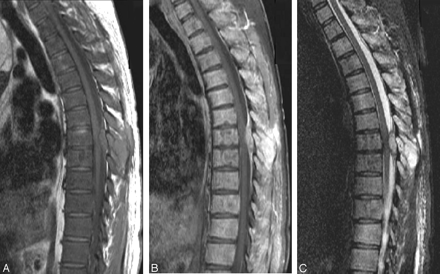
The patient was placed on vincristine and procarbazine but showed no response. One month after confirmation of the bone metastases, the patient developed spinal cord compression at T5 due to new epidural metastases (Fig 8A–C). This was soon followed by marrow failure and death.
The sagittal T1-weighted (A) image demonstrates abnormal diffuse low signal intensity of the vertebral body consistent with replacement of the normal fatty marrow by tumor cells as well as interval development of a posterior epidural mass compressing the cord. Sagittal T1-weighted postgadolinium with fat saturation (B) image demonstrates intense enhancement of the epidural mass with a dural tail sign as well as diffuse enhancement of the vertebral bodies. Sagittal T2-weighted with fat saturation (C) image demonstrates the epidural mass having homogeneous increased signal intensity with subtle but diffuse increased signal intensity of the vertebral body.
Discussion
Extraneural metastases from primary brain tumors are rare (1). The most common glioma to metastasize is glioblastoma multiforme, followed by medulloblastoma and ependymoma. An oligodendroglioma metastasizes very infrequently. In most cases (96% in one study) (2), extraneural metastasis occurred after surgical excision of the primary tumor.
There are several reports of local infiltration of the meninges as well as drop metastases to the spinal cord. Cases of primary oligodendrogliomas of the spinal cord are also described. Extraneural metastasis of oligodendroglioma occurs even more infrequently–our review of the literature found 30 reported cases since 1951, when the first case of metastatic oligodendroglioma was described (3).
Of these 30 cases (1, 4–10), 19 involved metastases to the bone marrow—usually diffuse metastases involving several vertebral bodies. Garner et al (1) stated that there were 6 reported cases, including theirs, in which bony metastases presented with hematologic abnormalities suggestive of diffuse infiltration of the bone marrow (pancytopenia, leukoerythroblastic anemia). On the basis of these findings, our patient represents only the seventh case in which diffuse metastases presented with a hematologic dysfunction (pancytopenia).
Macdonald et al (4) described 2 distinct patterns of spread of oligodendroglioma. Pattern 1 (local spread) is typified by metastases first appearing in the surrounding scalp or in ipsilateral cervical lymph nodes after multiple craniotomies. The tumor may subsequently disseminate to other organs. In pattern 2 (hematogenous spread), patients initially present with multiple bone metastases at the outset after craniotomy and failure of multimodal therapy. Garner et al (1) proposed adding a third category (cerebrospinal fluid spread) describing spread via the cerebrospinal fluid pathways, resulting in intradural deposits.
Our patient fits well into pattern 2 because he presented with widespread bony metastases after 3 previous craniotomies. However, this patient did not undergo radiation therapy or a full course of chemotherapy as did previous cases reported by Macdonald et al (4). Our patient is very similar to a case reported by Garner et al (1), in that diffuse bony metastases occurred without prior chemotherapy or radiation therapy.
The reasons for the rare occurrence of extraneural metastasis in primary tumors are not clear (11). Initial theories such as a lack of lymphatics in the brain, inability of neural tissue to grow outside of the central nervous system, and collapse of thin-walled cerebral veins by advancing tumor have been generally discredited. Currently, the most popular theories include the idea that brain tumors manifest earlier in their course and therefore have less time for the development of metastases. Another theory states the intracerebral environment is not sufficiently hostile to select out metastatic clones. There is relatively little connective tissue stroma in the brain versus the rest of the body. Clones are thus not selected for the ability to invade fibrous connective tissue and are thus not suited to invade extracranial tissues. The role of the blood-brain barrier is also unclear, though it does seem to provide a rate-limiting step in metastasis. This is evidenced by the extremely low rate of extraneural metastases in the absence of craniotomy or ventriculoperitoneal shunt surgery, as noted previously.
One difficulty in our review of the literature is that the exact histologic diagnosis of oligodendroglioma is still debatable. Several pathologists reading the same sample may differ on the diagnosis of oligodendroglioma versus astrocytoma. This possibility explains the wide discrepancy in the percentage of oligodendrogliomas out of all primary brain tumors (5%–33%) (12, 13). This is further complicated by oligoastrocytomas—mixed tumors with both oligodendroglial and astrocytic components. In addition, there are no specific markers available for oligodendroglioma.
This difficulty is highlighted in the article by Ordonez et al (14), who reported 2 cases counted by them as oligodendroglioma metastatic to the bone that were initially reported by Eade and Urich (15). However, by direct review of the article by Ordonez et al, we found that the biopsy of the bone in the first tumor showed exclusively undifferentiated cells, whereas the primary brain tumor was mixed astrocytoma and oligodendroglioma. In the second case, we found no mention of bony metastases and the primary was again a mixed tumor. Similar lack of clarity about exact histology was also apparent in the case reported by Jellinger et al (16). These reports exemplify the difficulty in a comprehensive review of the literature because the diagnoses are not always clear and histologic criteria have undergone changes.
For this reason, many of the previously reported cases of metastatic oligodendroglioma must be viewed with some suspicion. A number of cases were counted inappropriately in the past, and the exact number of cases of metastatic pure oligodendroglioma as well as the number of distant metastases remains unclear, but they are certainly very rare.
In summary, extraneural metastases with oligodendroglioma are very rare but certainly can occur. There are 3 types of spread: local, hematogenous, and via the cerebrospinal fluid. Hematogenous spread could lead to diffuse bone marrow infiltration resulting in hematologic dysfunction such as pancytopenia. In our patient, as in all cases in the literature, bony metastases occurred after prior craniotomy. The role of prior chemotherapy or radiation therapy is less clear, as we describe previously.
References
- Received August 30, 2004.
- Accepted after revision January 20, 2005.
- Copyright © American Society of Neuroradiology