Abstract
Summary: Carotid basilar anastomoses can occasionally persist beyond the embryonic period. These anomalies are most often incidentally detected in adulthood, during workups for unrelated pathologies. Persistence of the proatlantal intersegmental arteries is a rare form of primitive carotid-basilar anastomoses. Bilateral proatlantal inter- segmental arteries are an extremely rare occurrence, of which only 3 cases have been reported in the literature. An analysis of vascular anomalies associated with Galen’s vein malformations revealed 3 children in whom persistence of type II proatlantal arteries was seen. These included one child in whom proatlantal arteries were persistent bilaterally. We report the clinical and angiographic findings and discuss the embryologic and therapeutic implications of this unique association.
The persistent proatlantal artery is a well-recognized communication between the carotid and vertebrobasilar system. This communicating channel plays a critical part in irrigating the posterior circulatory bed in a developing embryo before the vertebrobasilar system fully develops. The proatlantal artery usually involutes by the 7- to 12-mm embryonic stage. Persistence of these channels is a well-recognized anomaly. More than 40 cases of such persistent communication have been described in the literature. However, persistence of bilateral proatlantal arteries is extremely rare (1, 2), and the exact incidence is not known. The proatlantal artery is commonly associated with other anomalies such as absent vertebral arteries (3, 4), vertebral artery hypoplasia, and an increased incidence of intracranial aneurysms (2). We report a case of bilateral type II proatlantal arteries and 2 cases of unilateral proatlantal arteries associated with the Galen’s vein malformation. To the best of our knowledge, no such rare and interesting association is described in the literature. Most often proatlantal arteries are identified incidentally. They may be of clinical significance in certain groups of patients. We present this case with a description of the embryology of this dual pathology and consider the hemodynamic implications of the presence of the proatlantal arteries and Galen’s vein malformation together.
Case Reports
Case 1
A 6-year-old girl of nonconsanguineous parents presented with a history of gradual proptosis of both eyes and prominence of the forehead since 4 years of age. On examination, she had no neurologic deficit and all biochemical parameters were within normal limits. CT of the head showed mild ventriculomegaly and prominent enhancing vessels in the perimesencephalic region and in the cistern of the velum interpositum. A dilated enhancing venous sac was also noted below the splenium of the corpus callosum, which continued posteriorly and joined a prominent superior sagittal sinus. Enhancing enlarged vessels were also seen in the cortical surface. The cavernous sinuses were enlarged bilaterally. Both superior and inferior ophthalmic veins were enlarged and tortuous. No parenchymal lesion was seen.
Selective bilateral internal carotid artery, external carotid artery, and vertebral angiograms showed the presence of a bilateral proatlantal artery with Galen’s vein malformation. Proatlantal arteries are schematically shown in Fig 1.
Schematic diagram of the lateral and anteroposterior views show the origin of the proatlantal artery from the external carotid artery at the second vertebral body level. Occipital artery origin from proatlantal artery is seen.
A Galen’s vein fistula was fed by thalamoperforating arteries, medial posterior choroidal arteries, and quadrigeminal arteries from both the right and left sides (Fig 2). The right perisplenial branch of the anterior cerebral artery and a branch of the left distal middle cerebral artery were also supplying the fistula (Fig 3). Bilateral posterior communicating arteries were enlarged to supply the fistula from the carotid arteries (Figs 2, 4). The superior sagittal sinus was not opacified from anterior circulation. Both middle cerebral and anterior cerebral arteries were draining via enlarged superficial cortical veins to the cavernous sinus and then via superior and inferior ophthalmic veins and the pterygoid plexus of veins. Engorged veins were seen in the falcine and tentorial leaves.
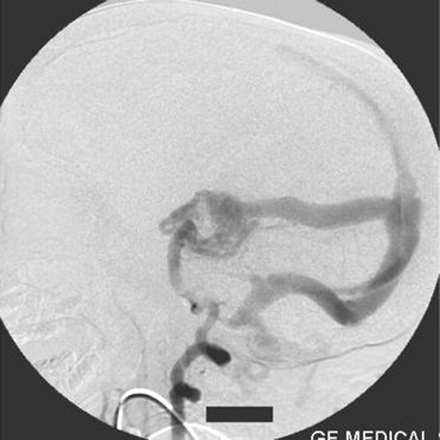
Right vertebral artery injection lateral view angiogram shows filling of the Galen’s vein fistula and the falcine sinus.
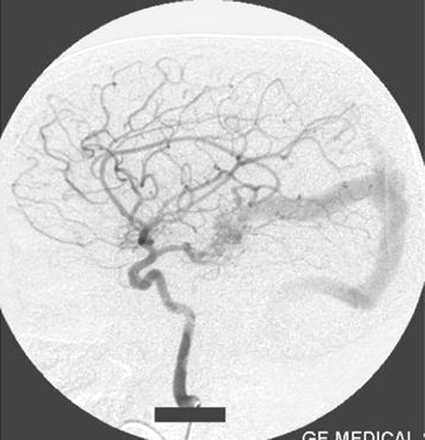
Right internal carotid artery injection lateral view angiogram shows the filling of the fistula through the posterior communicating artery.
Left internal carotid artery injection lateral view angiogram shows the filling of the fistula through the posterior communicating artery.
A straight sinus was absent, and the venous sac of the Galen’s vein fistula was draining via an abnormal falcine sinus to the superior sagittal sinus (Figs 2, 5). There was evidence of retrograde flow in the superior sagittal sinus from the posterior circulation. The deep venous system was not opacified. The right transverse and sigmoid sinuses were absent. Posterior circulation was draining mainly via the left transverse and sigmoid sinuses and partially via retrograde flow to the cavernous sinus. Both internal jugular veins were not opacified. The left sigmoid sinus was stenosed at its caudal end and was draining into the suboccipital and vertebral venous plexus.
Left vertebral artery injection lateral view angiogram shows the fistula filling from the thalamoperforator arteries.
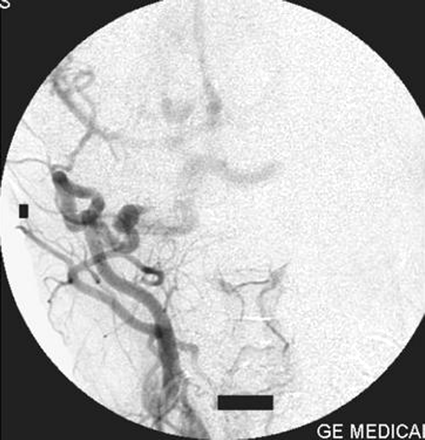
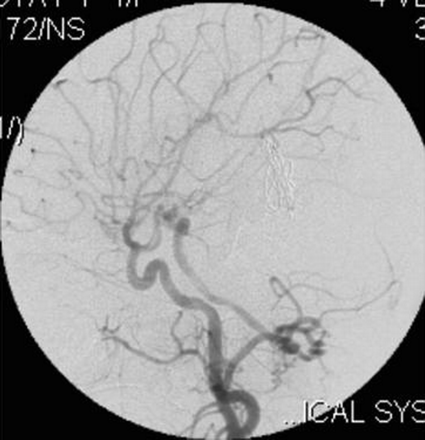
On the right external carotid artery angiogram, the carotid-basilar anastomotic vessel was seen to arise from the external carotid artery at the second cervical vertebral body (C2) level. The vessel coursed dorsally and joined the vertebral artery in the suboccipital space (Figs 6, 7). Contrast medium was seen to opacify the fistulous site and was draining via the left transverse sinus.
Right external carotid artery injection lateral view angiogram shows the proatlantal artery.
Right external artery injection anteroposterior view angiogram shows the opacification of the proatlantal artery of the right side.
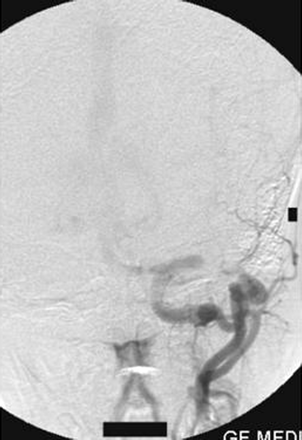
On the left external carotid artery angiogram, the origin of the carotid basilar anastomotic vessel arose at the C2 level from the external carotid artery, which coursed dorsally to join the vertebral artery in the suboccipital space (Figs 8, 9). On either external carotid artery injection angiogram, intracranial opacification was minimal because of wash-in from the contralateral channel.
Left external carotid artery injection lateral view angiogram shows the filling of the vertebral artery via the left proatlantal artery.
Left external carotid artery injection anteroposterior view angiogram shows the proatlantal artery and the opacification of the vertebral artery.
Therefore in this patient, the bilateral proatlantal arteries were associated with other anomalies such as absent right, left, or both straight sinuses; absent right transverse and sigmoid sinuses; absent internal jugular veins on both sides; and the presence of an abnormal falcine sinus, which connected the venous sac of the fistula of Galen’s vein with the superior sagittal sinus.
Case 2
The second case is a girl (1 year 2 months of age) who presented with a history of rapidly enlarging head size and a prominence of veins on the forehead. Angiography showed a left proatlantal artery of external carotid artery origin associated with a mural-type of Galen’s vein malformation that was supplied by the left anterior choroidal artery and thalamoperforators of the left posterior cerebral artery. The venous drainage was to the transverse sinus with some retrograde flow to the superior sagittal sinus. The origin and course of the proatlantal artery were the same as in case 1, and the occipital artery took its origin from the proatlantal artery (Fig 10).
Right common carotid artery injection angiogram shows the origin of the proatlantal artery and the filling of the vertebral artery (case 2).
Case 3
The third case was a 3-day-old male neonate who was diagnosed as having Galen’s vein malformation by antenatal sonography. The patient also had patent ductus arteriosus and presented with cardiac failure at birth, which was controlled medically. Angiography in this case revealed a left proatlantal artery of external carotid artery origin along with a mural-type of Galen’s vein malformation. The proatlantal artery revealed the same features as seen in case 2.
Discussion
Persistence of fetal carotid-vertebrobasilar anastomotic channels into adult life is not uncommon, the most frequent being a persistent trigeminal artery (5). Other proximal anastomoses like the otic artery and the hypoglossal artery are less frequent as is the persistent proatlantal artery (6). Gottschan first described the persistent proatlantal artery at autopsy in the 19th century. Since their first being named by Padget (7), bilateral proatlantal intersegmental arteries are exceedingly rarely reported in the literature. To the best of our knowledge, ours is the first report of bilateral proatlantal arteries in association with Galen’s vein malformation.
Current understanding of neurovascular embryology is derived from the contribution of Dorcas Padget (8). At the 3-mm embryonic stage (24 days), specific cranial vessels become apparent. At this stage, the neural tube is still open and the primitive internal carotid artery originates from the first aortic arch. The hindbrain is fed by the trigeminal artery, also a branch of the first aortic arch. By the 4-mm (28 days) embryonic stage, the first and second aortic arches regress, leaving the internal carotid artery supplied by paired dorsal aortas. The internal carotid artery passes up and divides into the caudal and cranial branches. The caudal branch of the internal carotid artery establishes connection with the posterior circulation via the mesencephalic branches. At this stage, a pair of longitudinal neural arteries develops at the base of the hindbrain, dorsal and parallel to the internal carotid arteries. Blood reaches them from dorsal aortas and carotid systems via a number of communicating branches. They are named “trigeminal,” “otic,” and “hypoglossal” arteries as determined by their relationship with the trigeminal ganglion, otic vesicle, and hypoglossal nerve. At the same time, in the 4- to 5-mm embryonic stage, each individual somite in the embryo receives blood supply from a single branch of paired dorsal aortas and innervation from a single nerve. The artery between the occipital somite and the cervical somite accompanies the first cervical nerve and provides communication between the forming carotid and vertebral circulation (7). Padget (7) named this artery the “proatlantal intersegmental artery,” because it was cranial to the first cervical segment.
At the 5- to 6-mm (29 days) embryonic stage, the caudal branch of the internal carotid artery communicates with the ipsilateral longitudinal neural artery, which later becomes the definitive posterior communicating artery. As the blood reaches via this communication and the proatlantal intersegmental artery, the other 3 communications regress. The otic artery regresses first, followed by the hypoglossal artery, and lastly the trigeminal artery. The proatlantal intersegmental artery becomes the major blood supply to the posterior circulation until the definitive vertebral arteries form from the sixth intersegmental arteries at the 12-mm embryonic stage, by which time 2 longitudinal arteries fuse to form the basilar artery. The proatlantal artery usually involutes by the 12- to 14-mm embryonic stage. Rarely, it fails to regress and persists into adult life. The proatlantal artery is of 2 types: Type I corresponds to the first segmental artery and arises from the cervical part of the internal carotid artery. Type II arises from the external carotid artery and corresponds to the second segmental artery (9). The evolution of the proatlantal artery is not very clear. It is thought to contribute to the horizontal or suboccipital segment of the vertebral artery and also to the occipital artery (10).
Now we briefly consider the embryologic aspect of the Galen’s vein malformation that is associated with the proatlantal arteries in our patient. Raybaud et al (11) extensively considered the embryonic aspect and anatomic features relating to the pathogenesis of Galen’s vein malformation. The precursor of this anomaly is a midline venous system that drains the choroid plexus, called the median prosencephalic vein of Markowski. According to Raybaud’s hypothesis, the arterial pattern of the feeders of a Galen’s vein aneurysm corresponds to that of the transient embryonic median prosencephalic vein of Markowski, a pattern that is reached by the sixth week of development but disappears when the prosencephalic vein changes at 11 weeks. Therefore, it has been inferred that a “true” Galen’s vein aneurysm is the result of an insult of unknown mechanism that occurs between 6 and 11 weeks of gestation.
Now, if we consider the gestational period when these malformations (proatlantal artery, aneurysm of Galen’s vein) occur, as in the present patient, the periods usually overlap. Therefore, we think some hemodynamic change that led to the persistence of the proatlantal artery in the present case may have occurred. Angiography in our case 1 revealed a high-flow fistula with significant steal from the posterior circulation as evidenced by the lack of filling in the posterior cerebral artery territory. So, we conclude that there might have been excess hemodynamic need, which led to the persistence of the proatlantal arteries bilaterally in our patient.
The actual incidence of proatlantal artery is probably higher than that reported because in most cases, the discovery is purely coincidental. A few authors also erroneously identified the proatlantal artery as a primitive hypoglossal or persistent first cervical intersegmental artery because of the similar angiographic configuration (12–14). Review of the literature shows that the proatlantal intersegmental artery arises either from the internal carotid artery or the external carotid artery at the second or third cervical vertebral body level. The proatlantal artery of external carotid artery origin ascends lateral to the transverse processes of the first cervical vertebra in the neck up to the medial aspect of the mastoid process to join the vertebral artery as seen in the anteroposterior view. In the lateral view, it ascends obliquely posterosuperiorly and passes at the level of the foramen transversarium of the atlas.
The proatlantal artery of internal carotid artery origin takes a more anteromedial course than that of external carotid artery origin. These 2 types of proatlantal arteries are distinct from the hypoglossal artery. The type I proatlantal artery, also known as the proatlantal intersegmental artery, arises from the internal carotid artery and traverses rostrally to enter the foramen magnum to join the vertebral artery. The type II proatlantal artery arising from the external carotid artery was also described as the first cervical intersegmental artery, which joins the vertebral artery before entering the foramen magnum. It remains more posterolateral in course than type I, but the first cervical intersegmental artery is different from the proatlantal artery of external carotid artery origin because the former passes through the foramen transversarium of the atlas. It is difficult to differentiate them in the lateral view, but in the anteroposterior view, the proatlantal artery ascends far lateral to the transverse process of the first cervical vertebra. The primitive hypoglossal artery is differentiated from the proatlantal artery by its straight upward course along the posterior aspect of the atlas before entering the hypoglossal canal and joining the basilar artery. The hypoglossal canal is enlarged in its presence (15). The primitive hypoglossal artery lacks the suboccipital horizontal sweep characteristic of the proatlantal artery.
In all our patients, the anomalous anastomotic vessel on both sides arises from the posterior surface of the external carotid arteries at the C2 vertebral body level, ascends obliquely across the atlas up to the suboccipital space, and in the lateral view, appears to traverse the foramen transversarium of the atlas. However, in the anteroposterior view, it ascends far lateral to the first transverse process up to the medial aspect of the mastoid to join the horizontal part of the vertebral artery in the suboccipital space. In cases of persistent proatlantal arteries, proximal vertebral arteries are hypoplastic in 46% (16). However, in all our patients, both vertebral arteries were entirely normal in their course and caliber. With the angiographic appearance of the anastomotic vessel between the carotid and vertebral arteries in our patients, we conclude that these are persistent bilateral type II proatlantal arteries.
Another important finding in our patients is that the proatlantal arteries gave rise to the occipital arteries. The proatlantal arteries of external carotid artery origin have a similar course to that of the occipital artery. Seeger et al (17) reported a case of histiocytoma of the right orbit in which the posterior circulation was opacified from a prominent anastomosis with the right occipital artery, with a striking resemblance to the proatlantal artery except for its smaller caliber. Now a doubt arises as to whether the proatlantal artery of external carotid artery origin is merely a hypertrophied collateral occipital artery when the vertebral artery is hypoplastic. However, in our patient, in the presence of normal bilateral vertebral arteries, the proatlantal arteries gave rise to the occipital arteries on both sides, which are smaller in caliber than the proatlantal arteries. This finding also supports the view suggested by Lasjaunias et al (10) that the distal part of the occipital artery might be derived from the proatlantal artery.
The second anomaly associated with our patient is the Galen’s vein aneurysm, which represents 30% of pediatric vascular malformations. More than 300 cases of such anomalies were reported in the literature since Jaeger et al (18) first described them in 1937.
In our patient, the arterial supply of the Galen’s vein fistula was identified as the bilateral posterior cerebral arteries, the thalamoperforators, the posterior choroidal artery, and the perisplenial branch of the right anterior cerebral artery. Visualization of the distal posterior cerebral circulation in the first patient was poor because of steal, the absent straight sinus, and the fistula draining via an abnormal falcine sinus along with the absence of the right transverse and sigmoid sinuses. The left sigmoid sinus was also occluded in its distal part and caused high venous pressure in the superior sagittal sinus, which led to the drainage of the anterior circulation via the superficial cortical veins and thence to the cavernous sinuses and ophthalmic veins.
The proatlantal artery, most commonly, is an incidental finding. However, it may be of clinical significance in our patient. Treatment of a Galen’s vein aneurysm with coiling of the venous sac carries a risk of hemorrhage in these patients due to the high pressure created by increased blood flow in the posterior circulation territory through the proatlantal arteries in addition to the vertebral arteries, or occlusion of the proatlantal arteries may result in an ischemic effect in the territory of posterior circulation.
Acknowledgments
We thank the director of the Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, Kerala, India, for his kind permission to present this report.
References
- Received June 13, 2003.
- Accepted after revision January 26, 2005.
- Copyright © American Society of Neuroradiology