Abstract
Summary: Osseous hemangiomas of the calvaria account for about 0.2% of bone neoplasms. We report a case of an extensive intraosseous cavernous hemangioma in a 46-year-old woman. MR imaging showed a mass in the right frontal bone with intra- and extracranial extension and a dural tail sign after gadopentetate dimeglumine administration, mimicking a meningioma in which the dural tail sign was due to a direct noninvasive superficial growth of the lesion.
Intraosseous hemangiomas usually occur as incidental findings in the skull or in the spine and represent slow-growing benign vascular malformations. Histopathologically, intraosseous hemangiomas are classified as venous, cavernous, or capillary type according to their vascular network (1). We report a case of intraosseous cavernous hemangioma of the skull with the presence of a dural tail sign.
Case Report
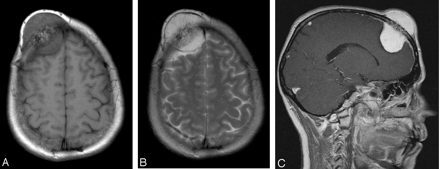
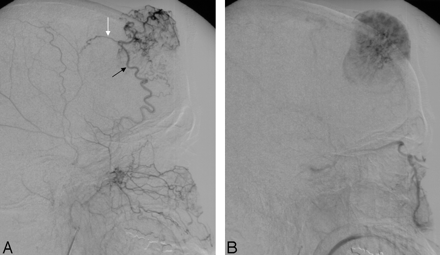
A 46-year-old woman was referred to our hospital with a gradually enlarging mass on her forehead. The mass was painless and did not produce any symptoms except cosmetic deformity. On physical examination, a bony hard painless mass was palpated. The overlying skin was mobile and normal in appearance. MR imaging demonstrated a mass in the right frontal bone with intra- and extracranial extension with an enhanced dural tail after gadopentetate dimeglumine injection (Fig 1A–C). Demonstrating the feeding vessels, conventional angiography showed a vascular mass that was supplied primarily by the right medial meningeal artery and by branches of the superficial temporal artery (Fig 2A, -B).
46-year-old woman with a gradually enlarging mass on her forehead. Noncontrast axial T1-weighted MR image (A) reveals a well-circumscribed mass, measuring 3 × 4 × 6 cm in the right frontal bone with intra- and extracranial extension. The lesion is well defined, and the signal intensity is of mixed intensity with some central hyperintensity and bone destruction. T2-weighted image (B) shows a lesion of high signal intensity with central areas of hypointensity. The lesion is well demarcated from the galea extracranially (outer uniform dark line) and the underlying dura intracranially (inner dark line). There is no surrounding edema. After gadopentetate dimeglumine administration (C), strong and homogeneous enhancement of the mass, relative enhancement of the bone marrow, and a dural tail sign are observed. The lesion extends intracranially and appears to compress the underlying brain, but without intraparenchymal extension. On the inner table in the parietal area, the small enhancing nodule corresponds to a vessel.
External carotid angiogram, lateral view, with midarterial (A) and capillary (B) phase, shows the rich vascularity of the tumor. The tumor shadow is fed by the right middle meningeal artery (black arrow) and by branches of the superficial temporal artery (white arrow).
We considered meningioma of the skull to be the most likely diagnosis because the lesion enhanced strongly after intravenous injection of contrast material and had a dural tail sign. Although meningioma was the initial impression, other skull tumors such as metastases or lymphoma were included in the differential diagnosis. The possibility of intraosseous cavernous hemangioma was not considered before surgery.
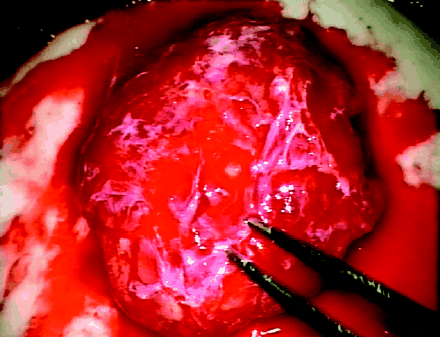
A right frontotemporal large craniotomy was performed. The tumor had a rich-vascular network, invaded the skull, and involved the dura. Bipolar coagulation of the dura followed by dural incision at the margins of the tumor was preformed. The tumor was solid and fairly well demarcated from the surrounding brain. We removed the firm, vascular tumor (Fig 3), applied dural substitute (Lyoplant, B. Braun Melsungen AG, Melsungen, Germany) for closing the dural defect, and performed cranioplasty.
Intraoperatively, the rich vascular network of the tumor is seen.
Gross pathologic examination showed a mass in the diploe (Fig 4A, -B; correlation with T1-weighted contrast-enhanced MR imaging). Histologic examination of the extraosseous portion of the mass (Fig 4C) revealed dilated blood-filled vessels, arranged in a diffuse haphazard pattern, with a single layer of endothelial cells. The abundant stroma was composed of loose connective tissue with scattered fat cells. In the intraosseous fraction of the lesion (Fig 4D), histomorphologically identical tissue was lying between plump bony trabeculae in irregularly shaped marrow spaces. Immunohistochemically, the endothelial cells expressed vimentin (Fig 4E) and CD34 (Fig 4F). At the site of the dural tail sign (Fig 4G; correlation with T1-weighted contrast-enhanced MR image), the dura was not invaded; instead, the lesion grew along the inner surface of the skull.
Correlation of T1-weighted contrast-enhanced MR image (A) with gross formalin-fixed specimen. Gross examination after formalin fixation shows a brown–red mass in the diploe, with radiated spicules and disruption of the outer and inner table (B); the bulk of the intra- and extracranial mass is missing as a result of the surgical excision technique. In the histomorphological representative section of the extraosseous fraction of the lesion (C), the fat cells (*) are seen as clear spaces without rimming; the vessels (§) are irregularly shaped dilated spaces lined by a single layer of inconspicuous endothelial cells (arrow) and often filled with erythrocytes (#) (hematoxylin and eosin, original magnification ×40). The vessels are arranged in a diffuse haphazard pattern. No signs of thrombosis or bleeding are seen. Intraosseous fraction (D) shows the hemangioma lying between plump bony trabeculae (double arrows) in irregularly shaped marrow spaces (hematoxylin and eosin, original magnification ×20). Immunohistochemically, the antibodies were directed against vimentin, which labels all mesenchymal cells (ie, all vessel walls and fat cells) (E), and CD34 (F), which labels endothelial cells (immunoperoxidase/diaminobenzidine method, original magnification ×100). In the correlation of the T1-weighted contrast-enhanced MR image with the histologic specimen at the site of dural tail sign (G), the dura is not invaded by the lesion; instead, the lesion grew along the inner surface of the skull. The van Gieson stain labels the cavernous hemangioma yellow–brown (mesenchymal component [μ]) and the collagenous fibers red (dura matter [$]). The arrow indicates the sharp delineation of the hemangioma (original magnification, ×20).
The findings at physical examination, bone scintigraphy, and abdominal sonography did not reveal other hemangiomas.
Discussion
Intraosseous hemangiomas are benign vascular anomalies of the bones. The most frequent sites of involvement are the vertebral column and the calvaria, particularly the frontal and parietal bones (2). They affect the diploe, causing an expansion of the outer table to a greater extent than the inner table and therefore producing a palpable lump. In contrast, in the described case of cavernous intraosseous hemangioma, there was also a large intracranial mass. Cavernous hemangiomas tend to be solitary lesions; however, multifocal cavernous hemangiomas have been reported (3).
Cavernous hemangioma of the calvaria commonly occurs in middle-aged women. There is no known familial association. It grows slowly and usually is asymptomatic; thus, most patients seek medical attention when the mass has reached 1–2 cm (2). A cavernous hemangioma causes compression on surrounding structures and may, in rare cases, present with epidural hematoma, proptosis, subarachnoid hemorrhage (3), or deformity.
Histopathologically, intraosseous hemangiomas are classified as venous, cavernous, or capillary type, according to their vascular network. The cavernous hemangioma is composed of large thin-walled vessels and sinusoids lined with a single layer of endothelium, whereas the capillary hemangioma is formed by a small fine vascular network filled with blood. Usually these 2 components are seen together as mixed hemangioma (1). Repeated bleeding induces hemosiderin and methemoglobin or deoxyhemoglobin deposits in fresh hemorrhages, which can be seen on CT and MR imaging. The increase in hemangioma size is probably due to repeated hemorrhage; however, in our patient, there were no signs of old or fresh hemorrhage. The enlargement of the described lesion was most probably due to the constant pressure of the blood on the malformed vessels, leading to a tortuous enlargement of the vessels themselves and, secondarily, leakage of serum from the malformed vessels, with consecutive reactive, reparative, and regressive changes in the interstitial space leading also to fat cells.
Skull radiographs usually show a lytic lesion with a sclerotic rim in a honeycomb or sunburst-like appearance (3). CT confirms the findings of plain film because of its excellent characterization of trabecular and cortical details (3, 4). On T1-weighted images, a nonhomogeneously hypo- to isointense mass is seen, and on T2-weighted images, a heterogeneous hyperintense mass is seen. The lesion enhances diffusely and heterogeneously after contrast medium administration (4), in contrast to our patient in whom the lesion enhanced strongly and homogeneously. Angiography may not show distinguishable feeder vessels (2); however, if feeders are identified, the middle meningeal and superficial temporal arteries are considered to be the main sources of the blood supply (3), which is in agreement with our findings.
The differential diagnosis in our case includes any slow-growing mass of the skull with normal overlying skin and with dural enhancement seen on MR imaging. The diagnosis of a meningioma was considered because the dural tail sign characterizes mainly meningiomas; furthermore, most scalp lumps are of benign origin, with meningiomas being the most frequent entities (5). Another diagnostic consideration was metastasis because most malignant scalp masses are of metastatic origin (6), and brain metastasis with the presence of a dural tail sign has been reported (7). Finally, the differential diagnosis of a lesion, combining the clinical and radiologic features of our case, includes lymphoma (3, 6, 8).
To the best of our knowledge, no case of intraosseous hemangioma with enhancement of the adjacent meninges has been described previously.
The dural tail sign may be caused by either direct tumor invasion or reactive meningeal changes (9, 10), and it is present in neoplastic and nonneoplastic lesions (9, 11). Furthermore, it is not specific for dural-based masses because it may be seen in association with both intraaxial and extraaxial lesions (10). In our patient, interestingly, the presence of a dural tail sign was not due to invasion or reactive changes, as one could expect, but the hemangioma grew along the surface of the dura mater without invasion and without producing reactive changes. The missing reactive changes are most likely due to the very slow growth of the lesion.
- Received August 31, 2004.
- Accepted after revision October 18, 2004.
- Copyright © American Society of Neuroradiology