Abstract
BACKGROUND AND PURPOSE: Multiple acute brain infarctions in both cerebral hemispheres usually suggest an embolic mechanism, particularly one of aortic or cardiac origin. The purpose of this study was to clarify the etiologic mechanisms and topographic features of bihemispheric infarctions depicted on diffusion-weighted imaging (DWI).
METHODS: Among 411 consecutive patients with ischemic stroke who underwent MR imaging in the acute phase, DWI showed bilateral infarctions in 19 (4.6%). In these patients, we analyzed the presence of carotid, aortic or cardiac embolic sources by using ultrasonography, cerebral angiography, and/or transesophageal echocardiography and evaluated the size and topographic distribution of the lesions. We assessed intracranial cross-flow through the anterior communicating artery, mainly on the basis of the anatomic information obatined from angiography or MR angiography.
RESULTS: Bilateral lesions were derived from cardiac and/or aortic embolic sources in 16 (84%) of 19 patients and appeared to originate from unilateral carotid diseases in three (16%). In nine (82%) of 11 patients with cardiac embolic sources, at least one large territorial or subcortical lesion was found in either hemisphere, whereas in all eight patients without a cardiac embolic source, the lesions were small and disseminated bilaterally.
CONCLUSION: Unilateral carotid lesions can cause bihemispheric infarctions through cross-flow in the anterior communicating artery. On DWI, small bihemispheric, disseminated lesions strongly suggest an artery-to-artery embolism. In such cases, aortic and carotid lesions should be assessed as potential embolic sources.
Diffusion-weighted imaging (DWI) has an excellent ability to depict recent small ischemic lesions, and it can depict multiple lesions in patients with acute stroke whose infarction is considered a single event from the clinical aspect ( 1, 2). According to MR imaging studies, 17–30% of patients with acute stroke have multiple lesions, and 1.4–6.1% have bihemispheric lesions in the anterior circulation ( 1–4). Bihemispheric infarctions strongly suggest the presence of embolic sources in the aorta or heart or the association of systemic hypercoagulopathy because of their distributions in multiple arterial territories. Baird et al ( 2) documented that multiple infarctions may result mainly from multiple emboli or the break up of a single embolus derived from aortic or cardiac disease. However, a single carotid artery lesion can also cause bihemispheric infarctions when the stenosed carotid artery supplies the blood flow to the contralateral hemisphere via intracranial cross-flow through the anterior communicating artery (AcoA) ( 4–6). To our knowledge, detailed mechanisms of bihemispheric infarctions, including artery-to-artery embolism of carotid origin, have not previously been investigated. The aim of the present study was to elucidate the etiologic mechanisms and topographic features of bihemispheric infarctions depicted on DWI.
Methods
Patient Population
Between January 1999 and March 2002, 449 patients were consecutively admitted to our department within 3 days after the onset of acute brain infarction. A total of 411 underwent MR imaging, including DWI, during the acute stage. Twenty (4.9%, 16 men and four women; mean age ± standard deviation, 73.3 ± 9.9 years) had a bihemispheric infarction in the anterior circulation, as shown on DWI. We excluded patients with systemic disease causing hypercoagulability, such as protein C deficiency or malignancy. Excluding one patient with antiphospholipid antibody syndrome, 19 (4.6%, 15 men and four women; mean age, 73.5 ±10.1 years) underwent further examination. Two patients also had infarctions in the posterior circulation and were included in the study. All patients provided informed consent was obtained before the study.
Evaluation of Risk Factors for Cerebrovascular Diseases
All patients underwent systemic examination, including determination of their complete blood cell count and blood chemistry and coagulation tests. Risk factors such as diabetes mellitus, hypercholesterolemia, habitual cigarette smoking, and cardiac disease were evaluated.
Cardiovascular Evaluation
We evaluated cardiac and aortic disease on the basis of results from several studies. Chest radiography, 12-lead electrocardiography, three-lead electrocardiographic monitoring for at least 24 hours after admission, and transthoracic echocardiography were performed in all 19 patients. Transesophageal echocardiography, including microbubble testing, was performed in eight patients. Cardiac diseases that create a risk of potential cardiac embolic sources include intracardiac thrombus, atrial fibrillation, recent myocardial infarction (<4 weeks), sick sinus syndrome, a patent foramen ovale with an interatrial septum aneurysm, mitral valve stenosis, prosthetic valves, and endocarditis ( 7). The risks of embolus from the thoracic aorta may be due to a mobile plaque or complicated lesion in the arch, which is defined as a lesion thicker than 4.0 mm, a 3.0-mm lesion with wall irregularity, or acoustic shadow in the arch of the thoracic aorta ( 8–10).
Cerebrovascular Evaluation
The extracranial carotid and vertebral arteries were evaluated with carotid ultrasonography in all 19 patients. Conventional cerebral angiography was also performed in nine patients. The intracranial cerebral arteries were evaluated with MR angiography in 10 patients, conventional cerebral angiography in three, and both studies in the remaining six patients. Stenotic lesions of the extracranial or intracranial arteries were defined as stenosis of at least 50% of the luminal diameter, as shown on Doppler ultrasonography, MR angiography, or conventional angiography ( 7). The degree of intracranial cross-flow through the AcoA was evaluated by using conventional angiography, or if not performed, with MR angiography. When the contralateral side ACA (ACA and MCA in some cases) was detected on conventional angiograms or when the AcoA was detected on MR angiograms, cross-flow via the AcoA was judged to exist. An aplastic A1 segment was defined 1) when the ACA was not detected on the ipsilateral carotid angiograms but was detected on the contralateral carotid angiograms or 2) when no A1 segment was depicted on MR angiograms. A hypoplastic A1 segment was defined when the ACA was detected on carotid angiograms and also on the contralateral carotid angiograms or when small A1 segment was present on MR angiograms ( 11, 12).
MR Imaging Evaluation
All patients underwent conventional MR imaging and DWI with echo-planner imaging (Magnetom Vision, 1.5 T, Siemens Medical Systems, Erlangen, Germany). Conventional imaging consisted of axial MR imaging, including T1-weighted (TR/TE = 630/14), T2-weighted (TR/TE = 5400/99), and fluid-attenuation inversion recovery (TR/TE/TI = 9000/105/2400) sequence. DWIs were simultaneously obtained by using a spin-echo planar imaging sequence. Diffusion gradients were applied in each of the x, y, and z directions with b = 1000 s/mm2. Criteria for the diagnosis of acute infarction on DWI were as follows: focal high signal intensities, a location or configuration not representing the normal anisotropy of diffusion, and a location or configuration not thought to represent a magnetic susceptibility artifact ( 13).
Topographic Distribution and Size of Lesions
We assessed the topography of the infarcts by using the mapping templates of Tatu et al ( 14). The territories of anterior circulation were defined as the distribution of the anterior cerebral artery (ACA) and the middle cerebral artery (MCA). When the ipsilateral internal carotid artery (ICA) supplied the posterior cerebral artery (PCA), the distribution of the PCA was also included in the anterior circulation. The watershed area was defined according to the templates of Bogousslavsky and Regli ( 15) and the atlas of Damasio ( 16).
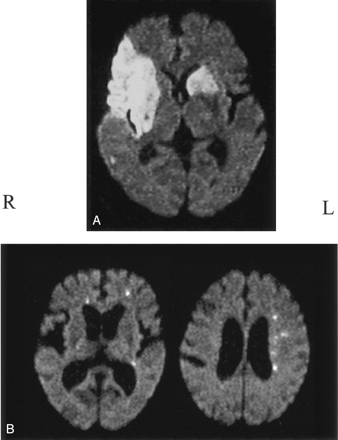
Modifying earlier methods ( 13, 17–19) based on the volume and distribution pattern of the hyperintense area on DWI, we classified infarctions into three types: 1) territorial infarction more than 15 mm in diameter, including more than one infarct with a continuous lesion from the cortex to the subcortex (Fig 1A, right side); 2) subcortical infarction more than 15 mm in diameter in the territory of deep perforating branches originating from the distal ICA or the MCA trunk (Fig 1A); and 3) disseminated small infarction less than 15 mm in diameter (Fig 1B). On the basis of the total volume of hyperintense areas on DWI, we defined the hemisphere with larger volume of the infarcts as the predominantly affected hemisphere, and that with the smaller volume was the subordinately affected hemisphere (Fig 1). The lesions were classified in blinded fashion with regard to the results of source identification, and vice versa.
Classification of infarction.
A, Right-sided infarction was defined as territorial; left sided, subcortical. Total volume of the infarcts was smaller on the left than right, which was defined as the predominantly affected hemisphere.
B, DWI shows small disseminated lesions. The predominantly affected hemisphere is the left side, and infarcts on the contralateral side are localized in the ACA area.
Classification According to Arterial and Cardiac Risks
On the basis of arterial and cardiac risks, we divided the patients into three groups, as follows: Group C included patients with cardiac disease; group CA, patients with carotid disease without cardiac disease; and group AO; patients with aortic arch lesions without cardiac or carotid artery disease.
Results
Seventeen patients had hypertension, eight had diabetes mellitus, eight had hypercholesterolemia, and three were habitual smokers. As shown in Table 1, 11 patients had cardiac disease, namely, chronic atrial fibrillation (n = 9), recent myocardial infarction (n = 2), mitral valve stenosis (n = 1), or a patent foramen ovale with deep venous thrombosis (n = 1), and they were classified into group C. At least one patient also had aortic lesions, though none had both cardiac and carotid artery lesions. Four patients had carotid artery lesions without cardiac disease and were classified into group CA. In this group, three patients underwent an evaluation of the aortic arch, and two were found to have aortic lesions. The remaining four had aortic lesions without cardiac disease or carotid disease and were classified into group AO. In all 19 patients, the manifestation of ischemic symptoms was abrupt, suggesting an embolic mechanism.
Patient characteristics
Table 2 shows the types of lesions and the affected vascular territories in both hemispheres in all patients. In group C, nine (81%) of 11 patients had at least one large territorial infarction (n = 6) or subcortical infarction (n = 3) in either hemisphere. Only two (19%) patients had small disseminated lesions alone in both hemispheres; in one (patient 6), aortic lesions were also found. In group AO and CA, all eight patients had only small disseminated lesions, which were bilateral.
Distribution pattern of infarcts
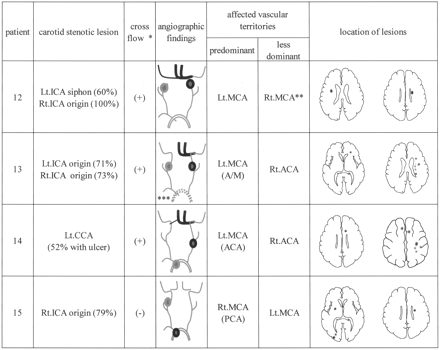
In all four patients in group CA, ICA stenosis was found on the side ipsilateral to the predominantly affected hemisphere. Figure 2 shows the angiographic and DWI findings in group CA. In patient 12, the contralateral carotid artery was occluded, and patients 13 and 14, the contralateral A1 segment was aplastic or hypoplastic; in these three patients, the stenotic ICA supplied both ACA areas via AcoA cross-flow. In patients 13 and 14, lesions in the contralateral hemisphere were located in the ACA area. In patient 15, the stenotic ICA supplied the blood flow to only the ipsilateral hemisphere because of the absence of AcoA cross-flow. This patient also had aortic lesions as a possible embolic source; therefore, bilateral infarcts were derived from the aortic lesions instead of the ICA lesion. Thus, bilateral lesions are considered to have derived from cardiac and/or aortic embolic sources in 16 patients (all 15 patients in group C and group AO plus one of four patients in group CA) and appeared to have originated from unilateral carotid diseases in three patients (three of four patients in group CA).
Angiographic and DWI findings in four patients in group CA. A/M = watershed area between the ACA and MCA, Lt. = left, Rt. = right, gray circle = carotid stenotic lesion or complicated lesion in the arch; black circle = culprit lesion; asterisk = cross-flow, intracranial cross-flow through the AcoA from the predominantly affected side to the contralateral side; double asterisk = right MCA area, which was supplied by the left ICA through the AcoA because the right ICA was completely occluded at the origin; and triple asterisk = aortic lesion, which remains unknown because of the lack of transesophageal echocardiography.
Discussion
We detected acute infarctions in both cerebral hemispheres in the anterior circulation in 20 (4.9%) of 411 patients with stroke. Our prevalence of DWI-depicted bihemispheric infarctions was similar to that previously reported (1.4–6.1%) ( 1–4). Multiple infarctions are generally thought to be caused by an embolic mechanism ( 2). In cases of bihemispheric infarctions, cardiac disease or an aortic lesion is usually suspected as a possible embolic source. However, embolic sources of bihemispheric infarctions are seldom evaluated. Bogousslavsky et al ( 3) found a cardiac embolic source on one-half of their patients with bihemispheric infarctions.
Roh et al ( 4) suggested that unilateral carotid artery lesions may cause bihemispheric infarctions. In their study, five of 20 patients with bihemispheric infarctions had stenotic lesions in the unilateral ICA, which supplied the contralateral ACA through intracranial cross-flow via the AcoA. In these five patients, the bihemispheric infarctions were all located in the territories supplied by the stenotic ICA. Unfortunately, the association of cardiac disease or aortic lesions was not evaluated, and hence, the importance of ICA lesions in the etiologic mechanisms of bihemispheric infarctions remains unclear. In our study, three of four patients with unilateral ICA lesion had AcoA cross-flow from the stenotic ICA to the contralateral ACA (Fig 2). In these patients, the stenotic ICA supplied blood flow to the territories including the infarcts. (Fig 2). The development of bihemispheric infarctions was presumably attributed to an embolism originating from the unilateral carotid lesion.
We found cardiac disease in 58% of patients with bihemispheric infarctions, similar Bogousslavsky et al ( 3). At least one large territorial infarction or subcortical infarction was found in nine of 11 patients with cardiac disease, whereas such territorial or subcortical infarction was not found in patients without cardiac disease. In our eight patients with carotid or aortic lesions unaccompanied by cardiac disease, all lesions were small and scattered in both cerebral hemispheres in a disseminated manner. Such a pattern of small bihemispheric, disseminated lesions was found in two of 11 patients with cardiac disease; one of them also had aortic lesions other than cardiac disease. Kang et al ( 6) evaluated the lesion pattern of infarctions in 35 patients with acute stroke and ICA stenosis or occlusion by using DWI and reported that large territorial infarction was rare. Matsumoto et al ( 20) studied the size of infarctions in acute stroke using DWI and reported that lesions in patients with carotid lesions or aortic lesions were generally small and disseminated. Therefore, the pattern of small bihemispheric, disseminated lesions appears to strongly suggest the existence of carotid lesions or aortic lesions as the possible embolic sources rather than cardiac disease. The role of aortic lesions in mechanism of bihemispheric infarctions is seldom evaluated, most likely because aortic lesions are often occur concomitantly with cardiac disease or carotid lesions. However, in four of our 19 patients, aortic lesions were found solely and not associated with other embolic sources. If we extrapolate the results from our relatively small samples to the general population, this finding implies that aortic lesions cause at least 20% of all bihemispheric infarctions.
The present study had some limitations. First, the apparent diffusion coefficient on DWI remains low for 2–10 days after the onset of acute stroke ( 21, 22). Provided that stroke occurs repeatedly within a short interval, the first and subsequent infarctions are likely demonstrated as lesions that develop simultaneously on DWI. The possibility that such recurrent infarctions were included as bihemispheric infarctions in the present study could not be excluded completely. Second, the patients were classified into three groups according to the type of embolic source found. However, the classification was incomplete: In group C, the presence or absence of aortic lesions was unknown in 10 of 11 patients, though none had carotid lesions. In group CA, aortic lesions coexisted in two of three patients and were not evaluated in the other patient, though none of them had cardiac disease. Only in group AO was the association of other embolic sources completely excluded in all patients. This partial lack of information and the coexistence of two embolic sources in some patients should be taken into account when the present results are interpreted.
Conclusion
Despite the general belief that bihemispheric infarctions are caused mainly by cardiogenic embolism, such infarctions may occasionally result from an artery-to-artery embolism originating from aortic lesions or unilateral carotid artery lesions. Small bilateral, spotty lesions strongly suggest an artery-to-artery embolism originating from an aortic or unilateral carotid artery lesion. Cardiac disease, aortic lesions, and/or carotid lesions may concomitantly occur in a patient with bihemispheric infarctions. In such patients, the possible embolic source must be determined speculatively to select either anticoagulation or antiplatelet therapy to prevent recurrent attacks. Our results provide information useful in selecting such preventive therapies.
Footnotes
Supported in part by the Research Grant for Cardiovascular Diseases (14C-1) from the Ministry of Health, Labor and Welfare of Japan.
References
- Received April 22, 2004.
- Accepted after revision August 20, 2004.
- American Society of Neuroradiology