Abstract
Summary: Parkinsonism and portosystemic encephalopathy (PSE) are cerebral disorders associated with motor and neuropsychological dysfunctions that may occur in patients with chronic liver disease. We describe a patient with parkinsonism and neuroradiologic and 1H spectroscopic findings of PSE associated with a large congenital intrahepatic portosystemic venous shunt. Chronic liver disease was absent. After endovascular treatment, we documented a progressive reversal of parkinsonism and PSE on the basis of clinical, neuroradiologic, and spectroscopic criteria.
Parkinsonism and portosystemic encephalopathy (PSE) are reversible cerebral disorders associated with motor and neuropsychological dysfunctions that may develop in patients with portosystemic shunts secondary to chronic liver disease, surgery, or rarely, an isolated congenital intra- or extrahepatic portosystemic venous shunt (1–4). PSE patients may present symptoms of parkinsonism and show characteristic brain MR imaging and 1H spectroscopic findings, despite the cause of the shunt (2, 5–10). We describe a progressive reversal of parkinsonism and PSE following embolization of a congenital intrahepatic portosystemic venous shunt, with emphasis on brain MR imaging and 1H spectroscopic findings.
Case Report
A 48-year-old woman presented with a history of parkinsonism defined by the presence of progressive bradykinesia and rigidity over a 24-month period. There were no signs of PSE on clinical examination. Subclinical PSE was diagnosed on the basis of neuropsychological impairments by using the Wechsler Adult Intelligence Scale—Revised, subtests Block Design and Digit Symbol from the Wechsler Adult Intelligence Scale Manual. Laboratory studies at the time of our examination showed normal electrolytes. The hematocrit, white blood cell count, platelets, prothrombin time, bilirubins, albumin, alanine aminotrasferase, aspartate aminotransferase, and gamma-glutamyltransferase levels revealed no abnormalities. The electroencephalographic findings were unremarkable.
MR imaging and 1H MR spectroscopy were performed at 1.5 T. T1-weighted (TR = 400 ms; TE = 20 ms) images showed typical neuroradiologic findings of PSE characterized by bilateral and symmetrical hyperintensities in the substantia nigra, cerebral peduncle, subthalamic region, hemispheric white matter, globus pallidus, and putamen (Fig 1A–C).
Initial examination. T1-weighted MR images show bilateral and symmetrical hyperintensities in the substantia nigra and cerebral peduncle (A), subthalamic region and hemispheric white matter (B), and the globus pallidus and putamen (C).
The diagnosis of PSE was also based on 1H spectroscopic findings by using a stimulated-echo acquisition mode technique (TR = 1,500 ms; TE = 30 ms) with the volume of interest placed in the medial part of the occipital lobes. We decided to place the volume of interest in the medial part of the occipital lobes because this area contains gray and white matter and allows measurement of a volume of interest with good homogeneity of the magnetic field, as proposed by Ross et al (8). In addition, it has been shown that occipital gray and white matter exhibit essentially identical MR spectroscopic metabolite changes in PSE when compared with parietal white matter regions (8).
For the major resonances—n-acetylaspartate (NAA), choline (Cho), and myo-inositol (mI)—ratios of metabolites were obtained, with creatine (Cr) used as a reference. Because of the considerable overlap between glutamate and glutamine (the metabolite of interest in this article), two integrations of peak areas were obtained for each spectrum, from the coupled peaks defining beta, gamma-glutamine between 2.2 and 2.4 ppm and alpha-glutamine between 3.6 and 3.75 ppm as proposed by Ross et al (8). These integrals were referred to as Glx. To define PSE at 1H spectroscopy, quantitative criteria were selected on the basis of demonstrated normal ranges for mI/Cr and Cho/Cr and the magnitude of changes from these mean values observed in an earlier study (8): mI/Cr and Cho/Cr <2 SD below normal.
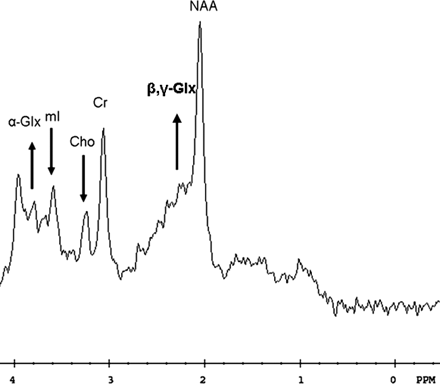
According to previously published parameters (8)—with normal ratios indicated in parenthesis—the pretreatment analysis showed decreased mI/Cr = 0.43 (0.51–0.63) and Cho/Cr = 0.43 (0.50–0.68) ratios (Fig 2).
Initial examination. 1H spectroscopy shows decreased mI/Cr and Cho/Cr ratios and an elevated alpha- and beta-, gamma-Glx/Cr peak areas ratios (arrows).
In the absence of hepatic failure, screening for a possible congenital shunt was performed and included an abdominal MR angiography examination. We detected a large congenital intrahepatic portosystemic venous shunt that communicated with the posterior division of the right branch of portal vein to the right hepatic vein, with an associated aneurysm (Fig 3A). It was further studied by digital angiography (Fig 3B). A percutaneous transhepatic sonographically guided catheterization of the left branch of portal vein was performed. The endovascular catheter was repositioned at the posterior division of the right branch of portal vein, and the portosystemic venous shunt and associated aneurysm were successfully occluded with the injection of n-butyl cyanoacrylate (Fig 3C).
Angio-MR examination (A) shows the right branch of portal vein (arrow), the intrahepatic aneurysm (arrowhead), and the right hepatic vein (smaller arrow). Portogram (B) shows the aneurysm (arrow) at the posterior division of the right branch of portal vein. Portogram after embolization (C) shows successful occlusion of the shunt and associated aneurysm.
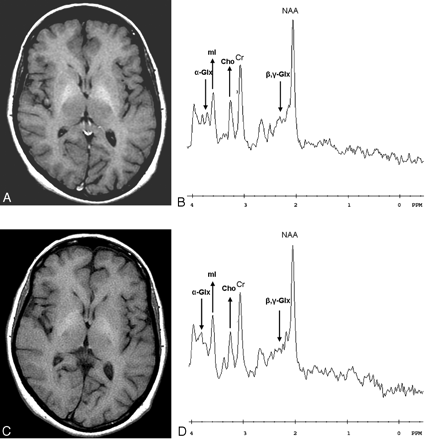
Progressive recovery of the parkinsonism was observed after treatment. MR imaging and 1H spectroscopy were performed 6 (Fig 4A and B) and 9 (Fig 4C and D) months later. After 6 months, the spectroscopic analysis already showed normalization of the mI/Cr and Cho/Cr ratios (mI/Cr = 0.61, Cho/Cr = 0.56; Fig 4B). Because of the relatively large standard deviations associated with determination of peak area ratios for glutamine, it is difficult to draw conclusions on the basis of one pretreatment spectrum and two post-treatment (6- and 9-month) spectra. These three consecutive spectra, however, showed a progressive decrease of the alpha- and beta, gamma-Glx/Cr peak areas ratios, as follows: alpha-Glx/Cr = 0.54, 0.48, and 0.30; beta, gamma-Glx/Cr = 0.79, 0.68, and 0.60 (Figs 2; 4B and D), respectively. In addition, the NAA/Cr ratios showed almost no variation, as follows: NAA/Cr = 1.42, 1.41, and 1.43, respectively.
Follow-up examinations after 6 (A, B) and 9 (C, D) months. T1-weighted MR images on the left show a progressive decrease of the symmetrical bilateral high signal intensity in the globus pallidus and putamen, and 1H spectroscopic measurements on the right show normalization of the mI/Cr and Cho/Cr ratios and a progressive decrease of the alpha- and beta, gamma-Glx/Cr peak areas ratios after 6 and 9 months (arrows).
The signal intensity abnormalities observed on T1-weighted images turned progressively into normal findings in a 9-month period (Fig 4A and C).
Discussion
We report the case of a patient with chronic and progressive symptoms of parkinsonism and neuroradiologic and 1H spectroscopic findings of PSE associated with a congenital intrahepatic portosystemic venous shunt, in the absence of any liver function abnormality. PSE is a distinct entity among parkinsonian syndromes (10), and neuropsychological tests showed subclinical disease in our patient. She presented with a progressive symmetrical akinetic-rigid syndrome with a notably absent resting tremor.
There was no history of alcoholism. Some features allow Parkinson disease as well as Parkinson-plus syndromes to be reasonably ruled out in this patient, such as absence of resting tremor and the relative symmetry of symptoms. Wilson disease was also ruled out. Similar parkinsonian symptoms have been described in the setting of hepatic failure and in spontaneous, surgical, or even congenital portosystemic shunt (2, 7, 11). MR imaging studies show, on T1-weighted images, bilateral and symmetrical hyperintensities in the globus pallidus, putamen, subthalamic region, substantia nigra, cerebral peduncle, and hemispheric white matter as in our report (2, 5, 7). Reasonable evidence points toward increased endogenous manganese deposition into the basal ganglia as a major cause of MR imaging hyperintensities observed in patients with portosystemic shunt (6, 7, 11). The involvement of the hemispheric white matter seems to be secondary to astrocytic edema (12).
Despite the large congenital intrahepatic portosystemic venous shunt, our patient did not present with any evidence of PSE on clinical examination. It has been shown that even in subclinical PSE, a specific pattern of cerebral metabolic changes exists in the brain, and this pattern can be noninvasively detected by using short-TE 1H spectroscopy (8). According to the literature, such striking and simultaneous changes in cerebral mI/Cr, Cho/Cr, and Glx/Cr ratios have not yet been described in association with other encephalopathies (8, 9). This high sensitivity is underscored by reports of few patients who exhibited mild 1H spectroscopic findings before developing PSE and in whom psychometric test results were notably normal (8). The markedly decreased mI signals in patients with PSE seem to reflect a disturbance of cell volume homeostasis in the brain, which may occur at preclinical stages of PSE in vivo. This disturbance of brain cell volume homeostasis may be attributed to an intracellular accumulation of glutamine in response to hyperammonemia. The 1H spectroscopic signal intensity for glutamine is increased with a compensatory loss of mI content in both preclinical and overt PSE. In light of the fact that glutamine formation from ammonia is a glial but not a neuronal process, glial cell swelling seems to be involved (12).
It has been shown that proper treatment of the underlying cause can revert the parkinsonian symptoms and the chain of metabolic abnormalities in PSE (2, 4, 9). In our report, the patient presented a striking clinical, neuroradiologic, and spectroscopic recovery over a 9-month period following endovascular therapy. It has been demonstrated that, despite the treatment of the causative factor, the reversal of both brain MR imaging and 1H spectroscopic abnormalities is slow, and the latter frequently reverses before the former (9), as in our case.
Conclusion
The congenital portosystemic venous shunt can be a potential treatable cause of acquired parkinsonism and PSE and must be suspected in every patient with brain MR imaging findings of PSE without evidence of liver failure. Brain MR imaging and 1H spectroscopy proved to be useful tools for the diagnosis and post-treatment follow-up in this situation, requiring months to demonstrate the reversibility of the process.
References
- Received October 17, 2003.
- Accepted after revision December 19, 2003.
- Copyright © American Society of Neuroradiology