Abstract
Summary: The site of lesions causing ataxia in Miller Fisher syndrome (MFS) remains in dispute. A 43-year-old man manifested rapidly progressive left-sided ptosis, bilateral abducens palsy, areflexia, and severe ataxia. Initial MR imaging showed confined lesions of the cauda equina with gadolinium enhancement. A diagnosis of MFS was made, and the patient underwent immunotherapy. His ophthalmoplegia disappeared, but other symptoms remained. Five months after onset, MR imaging disclosed lesions confined to the spinal posterior column, which were considered to result from involvement of posterior nerve roots of the cauda equina and to be responsible for his remaining severe ataxia.
Miller Fisher syndrome (MFS) is characterized by the clinical triad of ophthalmoplegia, ataxia, and areflexia (1), whereas its site of lesions, particularly of ataxia, is a matter of controversy (2–5) As a variant form of Guillain-Barré syndrome (GBS), MFS is generally thought to result from peripheral neuropathy (3, 6–8). Several MR imaging studies also have shown CNS abnormalities in MFS (9–13), which suggests that central lesions also are responsible for some clinical aspects of MFS. Here we report a patient with persistent severe ataxia in features of MFS whose MR imaging at the chronic stage showed lesions confined to the posterior column of the spinal cord.
Case Reports
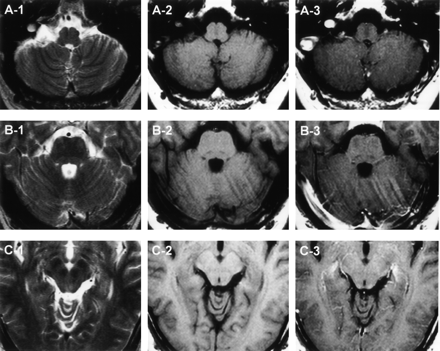
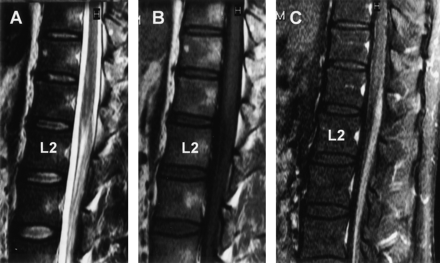
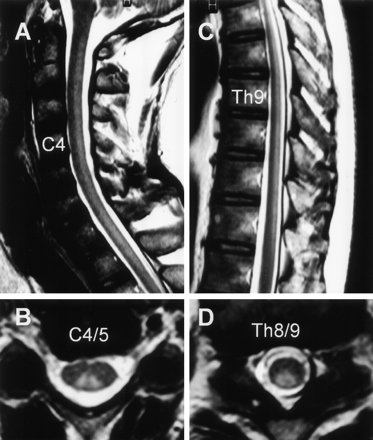
One month after suffering an upper respiratory tract infection with flulike symptoms, a 43-year-old man experienced dysesthesia and hypesthesia in all extremities followed by the development of diplopia and ataxic gait. On admission, he was alert and oriented. Speech was not dysarthric. Left-sided ptosis and bilateral abducens palsy were noted. Because of severe truncal and limb ataxia, he could not maintain an upright position without support and was unable to walk, even with assistance. Deep tendon reflexes were negative. In all extremities, there was a marked deterioration of position and vibratory sensation with modest dysesthesia and hypesthesia. Mild weakness of muscle strength was also noted. On CSF examination, the protein content was 134 mg/dL; the cell count was six cells/μL. Laboratory data, including CPK, were all within normal limits. Antibodies to gangliosides (GQ1b, GM1, GM1b, GM2, GD1a, GaNac-GD1a, GD1b, GD2, GT1a, and GT1b) and oligoclonal band were not detectable. The serum treponema pallidum hemagglutination test was negative. MR imaging performed on admission disclosed no apparent abnormalities in the brain, including the brain stem and cerebellum (Fig 1). At spinal MR imaging, however, gadolinium enhancement of the cauda equina was evident (Fig 2). A diagnosis of MFS was made, and the patient received 40 mg/kg/day of Gamma-globulin by intravenous administration for 5 days. Thereafter, his ophthalmoplegia disappeared, although severe ataxia, positional deficit, and lack of vibratory sensation persisted. He was seriously disabled, mainly because of truncal and limb ataxia. Somatosensory evoked-potential examination revealed no response to electric stimulation. Five months after onset, a repeat MR imaging study of his brain and spinal cord was performed and demonstrated on T2-weighed images hyperintense lesions confined to the spinal posterior column extending from the level of C1–T12 (Fig 3). No abnormalities were present on T1-weighed images or on the gadolinium-enhanced study.
MR imaging findings of the posterior fossa on admission. A, Axial view at the level of medulla and cerebellum. B, Axial view at the level of pons and cerebellum. C, Axial view at the level of midbrain. T2-weighted image, T1-weighted image, and gadolinium-enhanced images of each section indicated as 1, 2, and 3, respectively.
MR imaging findings of the sagittal spinal cord on admission at the lumbosacral level. A, T2-weighted image. B, T1-weighted image. C, Gadolinium-enhanced image. There is strong enhancement of the cauda equinae.
MR imaging findings on the spinal cord at 5 months after onset. Sagittal (A and C) and axial (B and D) views of T2-weighed images showing the hyperintense lesions confined to the spinal posterior column extending from the level of C1–Th12. A, Sagittal image of the cervical spine. B, Axial image at the level of C4/ C5. C, Sagittal image in the thoracic spine. D, Axial image at the level of T8/T9.
Discussion
Almost 50 years ago, Miller Fisher described a rare clinical syndrome with rapid evolving severe ataxia, ophthalmoplegia, and areflexia (MFS). Currently, MFS is generally considered a variant of GBS, but pathophysiology continues to be debated. The prognosis of MFS is usually benign (14,) but disabling or even fatal outcomes are occasionally found (15). Our patient presented the clinical triad of MFS with prior infection. CSF analysis showed a highly elevated protein content and a low cell count. Laboratory data including serologic tests and the initial MR imaging findings were not suggestive for other diseases such as neurosyphilis or multiple sclerosis, and we made a diagnosis of MFS. Indeed, immunotherapy abolished his cranial nerve symptoms; however, severe ataxia due to positional deficit and lack of vibratory sensation persisted, rendering him severely disabled and unable to lead an independent life.
In a recent study of 50 consecutive patients with MFS (14), the incidence of impaired vibratory or deep sensation was less than 20%, and all patients were minimally or not at all disabled 6 months after onset. With respect to the characteristic clinical features and prognosis of MFS, our patient was unusual because he continued to manifest a severe, unresolved deficit of deep sensations.
Ataxia is the most controversial symptom in MFS. Fisher interpreted it as the manifestation of an unusual peripheral neuron lesion, although he also raised the possibility that it might be of cerebellar origin (1). Other authors have supported his concept (3, 16, 17), and Kuwabara et al (18) recently suggested that MFS patients have dysfunction of the proprioceptive afferent system and their special sensory ataxia may be caused by the selective involvement of muscle spindle afferents. On the contrary, several authors proposed that ataxia in MFS is caused by the lesion of the cerebellar efferent or afferent pathway in the brain stem (4, 10, 11, 13, 19–21). On repeat MR imaging studies in our patient, although lesions of the cauda equina were initially found on the admission, lesions confined to the spinal posterior column were noted at 5 months after onset. On the basis of these MR imaging findings with anatomic considerations, we suggest that in our patient the anterograde degeneration of the posterior tracts in the spinal cord may occur secondarily to the initial involvement of posterior nerve roots of the cauda equina, resulting in his unresolved severe ataxia. In an autopsy analysis of ataxic forms of GBS, Richter reported degeneration of sensory roots, root entry zones, and posterior tracts (22). In some patients with GBS, MR imaging revealed involvement of posterior nerve roots, but not the posterior column, of the spinal cord (23, 24). With respect to lesion localization, debate continues on whether pure radicular involvement explains all the symptoms in MFS or whether ataxia is attributable to central and long-tract involvement. The imaging findings in our patient, who manifested involvement of the spinal posterior column, represent an important addition to the current understanding of pathophysiology of MFS.
Acknowledgments
We thank Dr. Nobuhiro Yuki of the Department of Neurology, Dokkyo University School of Medicine, Tochigi, Japan, for reviewing an earlier draft of the manuscript and providing critical suggestions.
References
- Received May 20, 2003.
- Accepted after revision June 24, 2003.
- Copyright © American Society of Neuroradiology