Abstract
BACKGROUND AND PURPOSE: Three-dimensional imaging and hemispheric volumetry are useful for the assessment of degenerative cortical atrophy. Our purpose was to determine the features of cortical atrophy in progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) by means of a hemispheric surface display generated with MR images.
METHODS: The extent of cortical atrophy was evaluated with automated MR hemispheric surface display and volumetry in 19 patients with PSP and 19 with CBD.
RESULTS: Most cortical regions were less atrophic in PSP than in CBD. The parietal lobe, paracentral regions, anterior middle frontal lobe, and posterior inferior frontal lobe were significantly more atrophic in CBD than in PSP, whereas the brainstem was significantly more atrophic in PSP. The mean hemisphere-to-intracranial volume ratio was significantly greater in patients with PSP (74.5%) than in those with CBD (71.4%), whereas the mean brainstem-to-intracranial volume ratio was significantly smaller in PSP (1.4%) than in CBD (1.6%). Asymmetry of hemispheric volume was significantly larger in the CBD group than in the PSP group.
CONCLUSION: Hemispheric surface display and volumetry are generally helpful and especially useful for the differentiation of PSP and CBD.
Progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) are neurodegenerative disorders that manifest with dementia and parkinsonism in middle-aged and elderly patients. The neurologic features of PSP are impaired ocular motility, pseudobulbar palsy, and axial dystonias, whereas CBD includes asymmetric rigidity of the limbs, myoclonus, the alien-limb sign, and localized cortical signs such as apraxia or cortical sensory loss. Because the clinical syndromes of typical PSP and CBD cases are distinct, it is usually not difficult to distinguish these two diseases if the subjects have typical symptoms. However, atypical cases with overlapping clinical and pathologic features are often seen. Several pathologic features, such as astrocytic lesions and ballooned neurons, differentiate PSP from CBD, although both diseases share several neuropathologic features including filamentous tau inclusions in neurons and glia and biochemical alterations in the tau protein (1, 2).
The patterns of brain atrophy and dysfunction of these two diseases can be visualized by means of neuroimaging. Radionuclear studies, which provide information about cortical dysfunction, are reportedly useful for differentiating these neurodegenerative disorders. MR imaging, which demonstrates structural changes, is useful for evaluating clinicopathologic correlations and establishing the diagnosis (3).
Several studies of single cases or case series have demonstrated the presence of high-grade cerebral atrophy in CBD; this may be bilateral and asymmetrical, involving the frontoparietal region contralateral to the side first and most severely affected (4, 5). Many groups have analyzed MR imaging of PSP (3, 6–10). Cordato et al (8) reported frontal atrophy in patients with PSP and found that frontal atrophy is correlated with behavioral changes. However, only a few groups have directly compared MR images of PSP with those of CBD. Soliveri et al (11, 12) demonstrated that MR findings of asymmetric frontoparietal atrophy in CBD and midbrain atrophy in PSP were the most consistent and useful aids in the clinical differentiation of the two diseases by visually rating atrophy of the midbrain and cortex. Yekhlef et al (13) also used visual inspection to evaluate cortical and midbrain atrophy and reported its diagnostic value. For visual inspection and additional quantitative measurement for evaluation of brain atrophy, Kitagaki et al (14–18) developed software that automatically extracts the brain matter from thin-sliced coronal MR images and generates 3D volume-rendered images of the cerebral hemisphere, brainstem, cerebellum, and calvaria. In previous studies, the precise anatomic location of cortical atrophy in frontotemporal dementia, CBD, and Alzheimer’s disease was determined by using these 3D reconstructed MR images (14–18), and the utility of this software was confirmed.
3D imaging and hemispheric volumetry are useful for the assessment of degenerative cortical atrophy. In this study, we used the 3D reconstruction of MR images to compare PSP with CBD to determine the extent of cortical atrophy in PSP.
Methods
Subjects
Nineteen patients with probable PSP diagnosed according to the clinical criteria established by the National Institute of Neurological Disorders and Stroke and the Society for Progressive Supranuclear Palsy International Workshop (19) were selected from among patients who were admitted to our hospital for examination of cognitive impairment. Another 19 patients with probable CBD who met the criteria of the CBD Multicenter Case-Control Study (CBDMCCS) (20) were sampled from the same cohort as those with PSP. All patients were matched on the basis of age, Mini-Mental State Examination (MMSE) score and Alzheimer’s disease Assessment Scale (ADAS) score. Patients with PSP consisted of 11 men and eight women with a mean age ± SD of 63.8 ± 6.3 years. Their respective MMSE and ADAS scores were 22.3 ± 5.3 and 20.8 ± 10.7. Patients with CBD consisted of 11 men and eight women with a mean age of 63.8 ± 6.7 years and respective MMSE and ADAS scores of 19.1 ± 6.0 and 26.1 ± 12.6. All subjects were right-handed.
Probable PSP requires the presence of a gradually progressive disorder with an onset at age 40 years or later, both vertical supranuclear gaze palsy and prominent postural instability occurring in the first year of onset, and no evidence of other diseases that could explain these features (20). Although clinical diagnostic criteria for CBD have not been established, Litvan et al (21) reported that their clinical diagnosis of CBD based on characteristic clinical features had good specificity. The CBDMCCS criteria require the presence of rigidity plus apraxia, cortical sensory loss, or alien-limb phenomena at some time during the course of the disease. Alternatively, the presence of moderate-to-marked limb rigidity accompanied by a fixed dystonic posture and spontaneous and reflex asymmetrical myoclonus can serve as inclusion criteria. The clinical features of the patients with PSP and those with CBD are summarized in Table 1. Written informed consent was obtained from all the patients or their relatives. The study protocol was approved by our institutional ethical committee.
Clinical features of patients with PSP and those with CBD
MR Acquisition, Image Processing, and Surface Display
All studies were performed on a 1.5-T MR imaging unit (Signa Horizon 5; GE Medical Systems, Milwaukee, WI) with a circularly polarized head coil functioning as both transmitter and receiver. Coronal 3D spoiled gradient-recalled (SPGR) images (field of view, 220 mm; matrix, 256 × 256; 124 × 1.5-mm contiguous sections; TR/TE/NEX, 14/3/2; flip angle, 20°) covering the whole calvaria were the source data. Voxel size was (220 mm/256)2 × 1.5 mm = 1.1 mm3.
Datasets of the SPGR images were directly transmitted from the MR imaging unit to a UNIX graphic workstation (Indigo 2, SGI; Mountain View, CA) and analyzed with 3D MR image-processing software (22). This software generated a 3D volume-rendered image of each structure; a two-dimensional reconstruction with arbitrary angles; and volumetric measurements of the bilateral cerebral hemisphere, brainstem, cerebellum, and CSF space (including ventricular systems). The posterior end of the whole brain and calvaria was manually set at the plane intersecting the occipitoatlantal junction. By using a mouse cursor to rotate the image, the rendered images of individual brain hemispheres could be seen at arbitrary angles.
Assessment of Cortical Atrophy
A 3D volume-rendered image of each cerebral hemisphere was displayed on a high-resolution color monitor. One neuroradiologist (K.I.) blinded to the clinical data and diagnosis rated regional cortical atrophy for each of the 66 cortical regions of the lateral and medial hemispheric surfaces into five categories: normal (nadir of the sulcus invisible with stacked gyri), minimal (nadir of the sulcus visible), mild (bottom of the sulcus widened), moderate (gyrus thinner than sulcus), and severe (very shrunken). The regions consisted of the orbitofrontal cortex, superior frontal gyrus (anterior and posterior, lateral and medial), middle frontal gyrus (anterior and posterior), inferior frontal gyrus (anterior and posterior), cingulate gyrus (anterior and posterior), precentral gyrus (medial and lateral), postcentral gyrus (medial and lateral), superior parietal lobule (anterior and posterior, lateral and medial), inferior parietal lobule (anterior and posterior), superior temporal gyrus (anterior and posterior), middle temporal gyrus (anterior and posterior), inferior temporal gyrus (anterior and posterior), parahippocampal gyrus (anterior and posterior), occipital gyri (anterior and posterior, lateral and medial), midbrain, and pons on either side. The score of the more affected side was considered representative for a given region, so that altogether 33 regions were evaluated.
In addition, the volumes of the right and left cerebral hemispheres, total cerebral volume, total intracranial volume, cerebellar volume, and the volume of the brainstem were determined. We also calculated total intracranial volume, hemispheric volume, cerebellar volume, brainstem volume, hemispheric volume-to-total intracranial volume ratio, cerebellar volume-to-total intracranial volume ratio, brainstem volume-to-total intracranial volume ratio, and asymmetry index. This index was calculated as the absolute value of (left hemispheric volume − right hemispheric volume)/(left hemispheric volume + right hemispheric volume).
We then assessed the relationship between clinical symptoms and cortical ratings. The cortical ratings for the positive and negative symptoms of apraxia, cortical sensory loss, alien limb, and myoclonus in the CBD group were compared.
Statistical Analysis
Differences between the PSP and CBD groups were analyzed with the Mann-Whitney U test for nonparametric ordinal data (atrophy rating). One-way analysis of variance was used for numerical data (volume). The level of statistical significance was set at P < .05 with Bonferroni correction. A stepwise discriminant-function analysis was used to identify the volumetry data most likely to help in distinguishing PSP from CBD.
Results
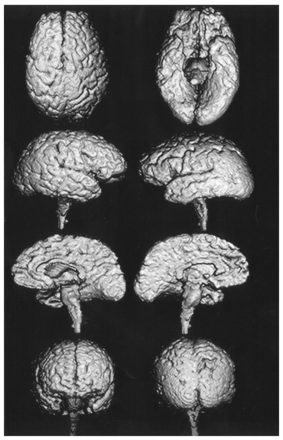
Patients in the PSP group had moderate atrophy in the midbrain. Mild atrophy was also observed in the frontal cortices but not in the parietal or occipital cortices (Fig 1).
3D volume-rendered images reconstructed from thin-section coronal 3D SPGR MR images (TR/TE, 14/3; field of view, 220 mm; matrix, 256 × 256; 124 × 1.5-mm contiguous sections; flip angle, 20°) show mild diffuse atrophy of cerebral cortex and moderate atrophy of brainstem in a 67-year-old woman with PSP (MMSE score of 21).
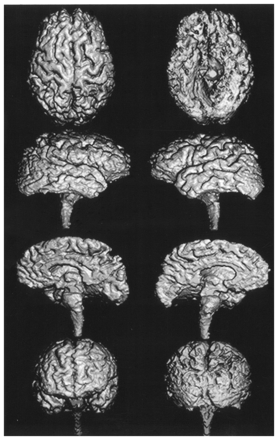
Focal atrophy affecting the frontal and parietal lobes was apparent on the hemispheric surface images of patients with CBD (Fig 2). The central area, especially the postcentral gyrus, was highly atrophic. In the frontal lobe, atrophy was concentrated in the lateral and medial surface of the superior frontal gyrus. The superior parietal lobule of the parietal lobe was especially severely affected. In contrast, atrophy of the temporal and occipital lobes was moderate.
3D volume-rendered images reconstructed from thin-section coronal 3D SPGR MR images (TR/TE, 14/3; field of view, 220 mm; matrix, 256 × 256; 124 × 1.5-mm contiguous section; flip angle, 20°) show severe frontoparietal atrophy in a 63-year-old woman with CBD (MMSE score of 19).
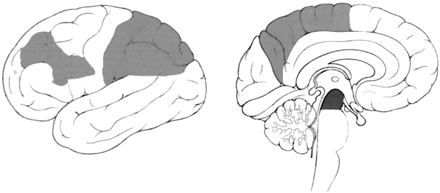
Table 2 shows the regions where cortical atrophy was significantly different for the two groups. Comparison of the PSP and CBD groups showed that 10 regions in the central areas, the frontal lobe, and the parietal lobe of patients with CBD were significantly atrophic, whereas the midbrain of patients with PSP was significantly atrophic. Figure 3 shows the significantly atrophic regions schematically. When an atrophy score of 3 was used as the cutoff point (CBD parietal atrophic rating >3, PSP midbrain atrophy rating >3), correct prediction occurred in 89% of the PSP cases and in 85% of the CBD cases.
Light gray and dark gray areas are regions where atrophy was rated significantly more severe in the CBD and PSP groups, respectively.
Mean visual regional atrophy ratings of the PSP and CBD groups
Table 3 shows the values for volumes and asymmetry index. The mean hemispheric volume and the mean hemispheric-to-total intracranial volume ratio were significantly smaller for CBD than for PSP. On the other hand, the mean brainstem volume-to-total intracranial volume ratio (relative volume) was significantly smaller for PSP than for CBD. The CBD and PSP group did not significantly differ in mean total intracranial volume, mean cerebellar volume, mean brainstem volume, or mean cerebellar-to-total intracranial volume ratio. The mean asymmetry index was significantly larger for the CBD group than for the PSP group.
Volumetric data for patients with PSP and those with CBD
Discriminant analysis used the following formulas: Classification score for PSP = (asymmetry index × 1.92) + (relative brainstem volume × 3908.6) + (relative right hemisphere volume × 837.3) − 168.7; Classification score for CBD = (asymmetry index × 2.147) + (relative brainstem volume × 4318.7) + (relative right hemisphere volume × 808.1) − 166.7. With this model, 79% of PSP and 74% of CBD cases were correctly predicted.
The exploratory comparison of atrophy ratings for the positive- and negative-symptom groups indicated that the anterior inferior frontal gyrus was significantly more atrophied in the alien-hand positive group than in the negative group. The positive and negative groups had no significant regional differences for other clinical symptoms.
Discussion
To identify the anatomic location of cortical atrophy in CBD, emphasis of pathologic examinations has been on the frontal, parietal, and central regions (23–26), whereas in PSP, the midbrain and pons are considered to be the pathologically affected regions (3). Although previous MR imaging studies of CBD have demonstrated asymmetric parietal and frontal cortical atrophy and asymmetric dilatation of the lateral ventricles (3, 4), the reports failed to specify the exact location of cortical atrophy. Making use of the advantages of MR imaging and computer technology, Kitagaki et al (18) were the first to document the extent of cortical atrophy in CBD with precise identification of its anatomic location in vivo. Test-retest reliability of the atrophy rating with this method was verified as good. Reliability was assessed by calculating the interclass correlation coefficients of two separate experimental ratings for 15 subjects who were randomly selected from the subject groups. Interclass coefficients ranged from 0.49 to 1.00, with a mean of 0.75 ± 0.14 (14). Relying on data from prior studies (14–18), we used the method of Kitagaki et al for our study of patients with PSP and those with CBD. Our rating results showed that atrophy was concentrated in the inferior frontal, paracentral, and parietal regions in patients with CBD and in the brainstem of patients with PSP.
Our study clearly demonstrated that parasagittal frontoparietal and paracentral cortical atrophy in CBD, which has often been emphasized in pathologic studies (23–26), was a useful MR sign for distinguishing CBD from PSP. Because asymmetry of atrophy in CBD was stressed in previous studies and also demonstrated in our volumetric study, it was found to be another useful sign for discriminating between CBD and PSP. A comparison of Parkinson disease and PSP showed that asymmetric hemispheric atrophy is a hallmark of CBD (4). Our findings verified the validity of this sign with surface-rendered images, while the volumetric asymmetry index also confirmed the prevalence of asymmetry of atrophy in CBD compared with PSP. Studies of functional neuroimaging approaches, such as positron emission tomography (PET) and single photon emission CT (SPECT), have demonstrated metabolic and perfusional impairment in both CBD and PSP (27–30). In CBD, metabolic and perfusional asymmetry and substantial reduction in the parietal, paracentral, and frontal lobes and in the basal ganglia and thalamus have been identified. In PSP, metabolism and perfusion is considerably reduced in the medial frontal lobe, basal ganglia and midbrain. Comparison of our two groups showed that CBD featured parietal and frontal atrophy, and PSP, only brainstem atrophy. This finding was noted because the frontal lobe in the PSP group was also atrophic, and the frontal lobes in the CBD group did not significantly differ except in the inferior frontal gyri.
As shown in our study, visual atrophy rating resulted in more accurate discrimination of the two diseases than did volumetric results. This may be due to the limitation of volumetry, which cannot be used to measure small cortical regions, while visual atrophy rating can be used to assess smaller regions. 3D reconstruction of the MR images is the first step in gross pathologic examination (i.e., inspection of the brain specimen before dissection). Detection of cortical atrophy with precise anatomic localization by the present system makes it possible to determine correlations between clinical symptoms and affected regions and to obtain useful additional evidence to support clinical diagnoses of the disease. In CBD, the basal ganglia is another affected locus, while the pallidus globes are a locus affected in PSP. However, our system is currently not capable of evaluating the internal structures, including the basal ganglia. However, in clinical situations, there is no need to evaluate basal ganglia atrophy for discriminating between these two diseases. Nevertheless, 3D reconstruction of MR images is a useful complementary tool for evaluating focal cortical atrophy in degenerative dementias and may facilitate the study of brain-behavior relationships and the differential diagnosis of dementia.
Our study demonstrated that only anterior inferior frontal cortical atrophy was correlated with the alien-hand symptom in CBD. Apraxia and cortical sensory loss in CBD may well be related to regional cortical atrophy, but we did not find any significantly atrophied regions related to those symptoms. This result may represent a limitation of morphologic MR imaging studies for the assessment of atrophy. Additional, more detailed regional volumetric measurements are needed to reach any definite conclusions.
Conclusion
Hemispheric surface display allows for external inspection of diseased brain and is useful for differentiating PSP and CBD. Asymmetry of hemispheric brain atrophy is a distinguishing feature of CBD and brainstem atrophy of PSP.
References
- Received November 19, 2003.
- Accepted after revision April 5, 2004.
- Copyright © American Society of Neuroradiology