Abstract
Summary: Management of arterial dissections can be particularly challenging. We report a case of vertebral artery dissection in which perfusion- and diffusion-weighted MR imaging findings suggested the presence of salvageable tissue, despite that the patient had symptoms for more than 40 hours. Direct access to the distal vascular territory was unattainable, and the presence of collateral circulation through an occipital vertebral anastomosis provided the only pathway to administer intraarterial thrombolysis. This case demonstrates that perfusion- and diffusion-weighted MR imaging can be instrumental in the selection of candidates for aggressive stroke therapy. Arterial anastomoses can provide alternate access to ischemic vascular beds and merit careful evaluation during intraarterial thrombolysis.
Intraarterial thrombolysis is a promising therapy for acute ischemic stroke. A randomized, controlled, open-label trial (PROACT-II) showed that patients undergoing intraarterial thrombolysis with recombinant pro-urokinase (pro-UK) within 6 hours of the onset of middle cerebral artery ischemia were 58% more likely to have slight or no neurologic disability after 3 months’ follow-up as compared with control subjects receiving heparin (1). Retrospective studies suggest that a broader therapeutic window is permissible for ischemic syndromes involving the vertebrobasilar circulation, given the devastating natural course of the disease (2, 3). Furthermore, the presence of viable brain tissue can be evaluated with perfusion- and diffusion-weighted MR imaging when patients present outside of the temporal boundaries traditionally advocated for intraarterial thrombolysis (4–7). Discrepancies between the size of regions exhibiting focal diffusion restriction and their related perfusion defect (ie, perfusion-diffusion “mismatch”) suggest the presence of tissue at risk of becoming infarcted unless regional perfusion is restored. It is thus conceivable that the finding of a mismatch genuinely indicates the presence of salvageable brain regardless of the duration of symptoms, opening avenues for the implementation of therapies aimed at achieving recanalization (4–7).
Nevertheless, intraarterial thrombolysis can be technically challenging. Access to the local area of occlusion may be hindered by unusual anatomy or markedly abnormal vasculature. One example is the complete occlusion of the vessel lumen by nonatheromatous lesions, such as craniocervical artery dissection (CCAD). Evidence suggests that brain ischemia during CCAD is mainly caused by artery to artery embolization, arising from the site of dissection. The ideal objective of the emergency treatment of CCAD is to restore circulation to the occluded distal vasculature without worsening the dissecting intramural hematoma proximally (8–11). Thrombolysis, either intravenous or intraarterial, is deemed feasible for acute ischemic stroke treatment associated with CCAD (<6 hours after symptom onset) (8–11). However, it may not be possible to deliver thrombolytic agents distally when the proximal end of the affected artery has been obliterated by the extrinsic compression of a mural hematoma. Here we describe a case of CCAD in which the presence of mismatch several hours after the onset of symptoms provided the grounds for proceeding with intraarterial thrombolysis. Although direct access to the affected circulatory site was unattainable, the presence of collateral circulation (occipital vertebral anastomosis) provided a pathway to perform intraarterial thrombolysis.
Case Report
A 34-year-old Asian woman was admitted to a local community hospital because of a 24-hour history of rapidly progressive gait difficulties and slurred speech. She was well the morning of her admission to another hospital but became somnolent and clumsy over the course of the day. The patient had a history of hypothyroidism and was treated with levothyroxine. She did not have any relevant cardiovascular risk factors. The patient had participated in Yoga classes involving neck flexion and extension exercises for several weeks before hospitalization. On initial physical examination, she was hemodynamically stable. She was lethargic but would easily awake and answer questions appropriately. The patient had bilateral nystagmus, and her gag reflex was absent. She was noted to have bilateral limb ataxia and was unable to stand and walk without assistance. Cranial MR imaging showed a basilar occlusion and scattered brain stem infarcts. Her condition was noted to deteriorate; therefore, she was transferred to our hospital.
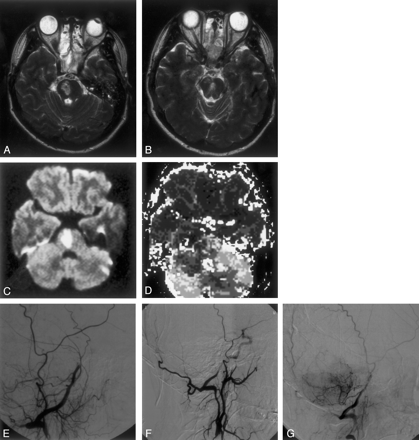
By the late evening, when the patient arrived at our institution, she was unarousable, with a dilated, unreactive left pupil and forced conjugated gaze deviation to the left. To vigorous stimulation she was noted to have a dense left hemiparesis. Her National Institutes of Health stroke scale (NIHSS) score was 20. She was urgently intubated and admitted to the neurologic critical care unit. Repeat MR imaging performed 39 hours after onset of symptoms revealed foci of hyperintense signal on T2-weighted images within the left thalamus, right midpons, and left side of the midbrain (Fig 1A and B). These lesions were hyperintense on diffusion-weighted images and hypointense on the apparent diffusion coefficient maps (Fig 1C). Perfusion-weighted imaging with time-to-peak maps showed patchy regions of oligemia involving the cerebellum and pons (Fig 1D). The comparison of the diffusion and perfusion findings revealed a perfusion-diffusion mismatch (lesion volumes measured manually were 1.6 and 24.5 mL for diffusion- and perfusion-weighted imaging, respectively).
A 34-year-old woman with sudden onset of ataxia and dysarthria was transferred to our institution because of neurologic degeneration 24 hours after the beginning of symptoms.
A and B, T2-weighted images obtained 39 hours after symptom onset, revealing foci of hyperintense signal involving the right side and paramedial regions of the midpons.
C and D, Diffusion-weighted image (C) also reveals foci of hyperintense signal. Apparent diffusion coefficient maps (not shown) ruled out T2 shine-through. Perfusion-weighted image (D) shows patchy regions of oligemia involving the cerebellum and pons. Comparison of C and D shows a perfusion-diffusion mismatch.
E and F, Oblique- (E) and lateral-view (F) sequential-phase digital subtraction angiograms obtained 43 hours after symptom onset show posterior circulation opacification through an occipital vertebral anastomosis. Note the abrupt end of the distal basilar artery and virtual absence of cerebellar blush.
G, Lateral-view digital subtraction angiogram of the vertebrobasilar circulation obtained after intraarterial thrombolysis with rt-PA. Although the distal basilar artery remains occluded, a substantial improvement of the cerebellar blush is appreciated.
Emergent digital subtraction angiography performed 43 hours after symptom onset revealed a hypoplastic and irregular left vertebral artery that ended in the left posterior inferior cerebellar artery. Injection of the right subclavian artery with an inflated blood pressure cuff demonstrated an occluded proximal right vertebral artery without cephalad reconstitution. The anterior circulation was unremarkable following injection of both common carotid arteries. The injection of the right common carotid artery, however, resulted in opacification of the distal right vertebral artery through a prominent occipital vertebral anastomosis. An abrupt occlusion of the basilar artery at the level of the posterior inferior cerebellar artery was thus observed (Fig 1E and F). It was concluded that the right vertebral artery had dissected proximally with subsequent embolism to the distal basilar artery, in accord with the MR imaging findings. Because of the proximal vertebral artery occlusion, it was thought that the only access to the distal basilar territory was through the right external carotid artery to the right vertebral artery via the knot of Bosniak. Using a Turbo Tracker 18 (Boston Scientific Corp, Natick, MA) and a Transcend 10 wire (Boston Scientific Corp), it was possible to navigate the catheter just beyond the knot of Bosniak. From this location, recombinant tissue plasminogen activator (rt-PA) was injected by using 5-mg aliquots every 10 minutes, to a total dose of 15 mg, resulting in partial improvement of the cerebellar blush bilaterally (Fig 1G).
The patient was readmitted to the neurologic critical care unit after the procedure. She received anticoagulation with IV heparin (aiming for a partial thromblastin time ratio of 2), and her blood pressure was increased with IV phenylephrine and normal saline, resulting in slow neurologic improvement to a NIHSS of 16. Follow-up MR imaging 5 days later showed improvement of the perfusion defect (volume was 18.5 mL) but no change of the diffusion abnormalities. The hospital course was complicated with hospital-acquired pneumonia, which responded well to antibiotics. The patient was discharged from the neurologic critical care unit in stable condition and received oral-pathway warfarin, which was continued for 6 months after her discharge. The patient had a satisfactory neurologic recovery after a short period of in-patient rehabilitation. She is now able to perform activities of daily living and can walk unassisted. Her eye movement abnormalities have resolved. She has marked residual limb spasticity (left greater than right), for which she is receiving physical therapy. She continues to have mild to moderate dysarthria. Her NIHSS and Rankin scores 6 months after intraarterial thrombolysis were 3 and 1, respectively. Follow-up angiography showed persistence of the right vertebral occlusion and patency of the occipital vertebral anastomosis.
Discussion
Herein we describe the administration of intraarterial thrombolysis several hours after the onset of symptoms in a patient with ischemic stroke due to CCAD. In this context, diffusion- and perfusion-weighted MR imaging are more sensitive than CT and conventional MR imaging for early detection of brain ischemia because these techniques disclose areas of infarction within a few hours after symptom onset in about 95% of patients (12). Perfusion-weighted imaging uses a gadolinium bolus to generate changes in signal intensity throughout the brain in a time-dependent fashion, paralleling cerebral blood flow. Perfusion-weighted imaging provides relative rather than absolute estimates of regional blood flow. Areas of conspicuous diffusion abnormality are considered irreversibly damaged, whereas perfusion defects can be reversible (4–7). Some patients may exhibit a perfusion-diffusion “mismatch,” which suggests the presence of salvageable brain tissue. Therefore, the information provided by the combination of diffusion- and perfusion-weighted imaging has relevant implications and may provide sensible grounds for the implementation of aggressive therapy regardless of the duration of symptoms (4–7).
Another relevant aspect of the reported case is that intraarterial thrombolysis was delivered through an occipital vertebral anastomosis, which provided the only access to the affected vascular territory. Also referred to as “knot” of Bosniak or suboccipital “carrefour” (ie, crossroads), the occipital vertebral anastomosis constitutes a network of arterial channels interconnecting the vertebral and external carotid systems through the occipital artery (13–15). The importance of such an arterial anastomotic network stems from the possibility of its opportune recruitment during brain ischemia (13, 15, 16). Sudden opening of an occipital vertebral anastomosis has been observed angiographically during the embolization of arteriovenous malformations, suggesting that their abrupt unveiling is mediated either by increased intraluminal pressures due to emboli or perhaps in response to vasoactive substances released by ischemic tissue (16). The presence of alternative circulatory pathways is essential for the survival of neural cells subjected to ischemia. In particular, the attendance of collateral blood flow to the posterior circulation, especially through patent posterior communicating arteries, appears to be associated with better neurologic outcomes after basilar artery intraarterial thrombolysis (17, 18). In addition to the knot of Bosniak, other named carotid basilar anastomoses include the persistent trigeminal, the hypoglossal, the otic, and the primitive proatlantal intersegmental arteries.
The present case also serves to expand the application of intraarterial thrombolysis for acute ischemic stroke due to CCAD. The use of IV thrombolysis with rt-PA has been described in small case series, mostly involving carotid artery dissections (8–10). In contrast, intraarterial thrombolysis appears to be favored by physicians confronting vertebrobasilar dissections. At least 10 patients with vertebrobasilar dissections, treated with intraarterial thrombolysis, have been reported in the literature (8, 11, 19–21). Of these cases, only three received intraarterial rt-PA and the remainder pro-UK. Although the clinical outcome was satisfactory in five patients, two patients died as consequence of progressive brain stem ischemia, in spite of the implemented therapy (8, 20). One obvious limitation of thrombolysis is the possibility of hemorrhage. More specifically, there is a justified concern for complications related to the extension of the arterial wall hematoma associated with CCAD (8–10). Nonetheless, such a regrettable complication appears to be scarce and perhaps represents “an entirely theoretical concern,” according to Schievink (10). Therefore, intraarterial thrombolysis is considered a reasonable option in patients with acute ischemic stroke associated with CCAD.
Technical difficulties may account for a small number of unsuccessful intraarterial thrombolysis cases. Such adverse circumstances reportedly interfered with the use of intraarterial thrombolysis in 1.6% of patients randomly selected to receive thrombolysis in the PROACT II study (1). Therefore, a conscientious effort to perform a thorough diagnostic angiographic study of the craniocervical circulation is essential before formulating an optimal intraarterial thrombolysis strategy. In our particular case, the injection of contrast medium in the unaffected anterior circulation proved its unmistakable value by exposing an unexpected route for intraarterial thrombolysis.
Our case also serves to illustrate another point of clinical significance, which is that outcome may not correlate with angiographic results. Although recanalization is considered one of the most relevant factors associated with neurologic improvement, it was only partially achieved in our patient. However, the presence of significant collateralization through unexpected anastomoses is in part responsible for the satisfactory clinical outcome.
Conclusion
We conclude that the assessment of acute brain ischemia with diffusion- and perfusion-weighted imaging may be advantageous regardless of the duration of symptoms. A detailed search for craniocervical anastomosis should be considered whenever intraarterial thrombolysis appears problematic because of technical limitations, particularly a complete nonthrombotic occlusion of the proximal end of the affected vessel. Furthermore, a judicious diagnostic exercise may provide the means for delivering specific therapy to the involved circulatory territory during acute stroke.
References
- Received December 16, 2002.
- Accepted after revision April 13, 2003.
- Copyright © American Society of Neuroradiology