Abstract
BACKGROUND AND PURPOSE: We retrospectively analyzed our results with Guglielmi detachable coils (GDCs) for the endovascular occlusion of acutely ruptured saccular cerebral aneurysms over 10 years.
METHODS: Between 1991–2000, 83 patients (mean age, 56.1 years) with aneurysmal subarachnoid hemorrhage were treated with endovascular GDCs. Patients with aneurysms due to trauma or dissection and those with mycotic or fusiform aneurysms were excluded. Mean follow-up in survivors was 19.1 months, and the mean Hunt-Hess grade at admission was 2.2. Angiographic follow-up was performed in 93% of surviving patients (mean interval, 11.6 months). The basilar caput (34 patients) and anterior communicating artery complex (19 patients) were most commonly treated.
RESULTS: Sixty-four patients (77%) had a Glasgow Outcome Scale score (GOS) of 4 or 5, nine (11%) had a score of 2 or 3, and 10 (12%) died. At follow-up, 24 patients (35%) had complete aneurysm occlusion, 18 (26%) had a dog-ear remnant, 24 (35%) had a residual neck, and two (3%) had residual aneurysm filling. No treated aneurysm rebled. Three patients required surgical repair after incomplete endovascular treatment. Two or more GDC occlusion procedures were required in 28 patients (34%). Major procedural complications occurred in two patients (2%), resulting in serious neurologic disability or death.
CONCLUSION: Endovascular treatment of ruptured cerebral aneurysms with GDCs has low morbidity, and it facilitates good overall outcomes in patients after subarachnoid hemorrhage. The short-term effectiveness of GDC occlusion in preventing aneurysmal rebleeding was excellent. Durability of the treatment in preventing long-term rebleeding as compared with direct surgical clipping warrants further study. Advances in device technology and technique may improve future outcomes.
Since the introduction of the Guglielmi detachable coil (GDC), the use of endovascular techniques in the treatment of ruptured cerebral aneurysms has increased substantially (1–17). Although precise indications for choosing surgical or endovascular repair for a ruptured cerebral aneurysm are lacking, most clinicians recognize a role for endovascular techniques in the management of certain patients. Direct GDC occlusion is the most effective technique for treating ruptured cerebral aneurysms in the current armamentarium of the endovascular surgeon. Over the 10 years during which GDCs have been used for aneurysm occlusion, substantial advances have been made in both endovascular instrumentation and technique.
The goal of aneurysm occlusion after subarachnoid hemorrhage (SAH), with either surgical or endovascular means, is the prevention of rebleeding from the aneurysm. Early aneurysm surgery after SAH has been proved highly effective in preventing early and long-term aneurysmal rebleeding (18–21). We analyzed our results with the use of GDCs for the endovascular occlusion of acutely ruptured saccular cerebral aneurysms by a single practitioner at one institution over the 10 years since the introduction of GDCs in 1991.
Methods
Clinical Material
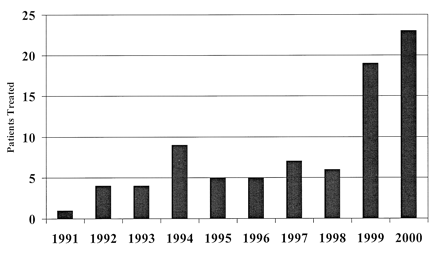
We retrospectively reviewed the medical records and angiograms of 83 consecutive patients treated with endovascular GDC occlusion for a saccular cerebral aneurysm after SAH from 1991 to 2000 (Fig 1). The treated aneurysm was believed to be the source of SAH in every case. All procedures were performed at a single institution, by a single endovascular surgeon (D.A.N.). Patients with aneurysms arising as a result of trauma or dissection were not considered, nor were patients with mycotic or fusiform aneurysms. Only patients in whom one or more GDCs were actually detached were considered. Patients who underwent GDC occlusion of aneurysms after incomplete previous surgical clip placement or incomplete previous GDC occlusion elsewhere were excluded. In 21 patients with multiple aneurysms, only the aneurysm thought most likely responsible for the SAH was considered for the purpose of this analysis.
Graph depicting patients with ruptured cerebral aneurysms treated with endovascular GDC occlusion by year.
In this series, patient selection for endovascular treatment rather than surgical repair of a ruptured cerebral aneurysm was difficult to accurately assess, because the basis for selection was frequently multifactorial, and it evolved over the 10 years of the study. Primary factors included a poor clinical grade, the presence of posterior circulation aneurysms, and the preferences of the patient and referring neurosurgeon. The study was reviewed and approved by the Mayo Clinic institutional review board.
Technique
Endovascular techniques varied on the basis of the size and three-dimensional morphology of the aneurysm and on the available technology at the time of treatment. Early in the series, approximately 50% of the procedures were performed with the patient under general anesthesia, and 50% were performed with the patient under conscious sedation. In addition, patients were routinely medicated with 10 mg of dexamethasone at the beginning of the procedure. Since 1994, all procedures have been performed with general anesthesia, and steroids have not been routinely used before or during the procedure. The typical technique was as follows: With a modified Seldinger technique, 6F arterial sheaths were placed in the femoral arteries bilaterally. A baseline activated clotting time (ACT) was obtained, and heparin was administered as an intravenous bolus to achieve an ACT approximately two times that of normal. ACTs were obtained every 20–30 minutes, and additional heparin was given as a bolus when it was necessary to maintain a therapeutic ACT. This protocol for anticoagulation was used throughout the 10 years of the study. A catheter was selectively placed in the brachiocephalic artery supplying the aneurysm with a variety of 5F or 6F delivery catheters. Multiple biplane digital subtraction angiographic (DSA) series were obtained to demonstrate the angiographic anatomy of the aneurysm. When the optimal angiographic projection was determined, selective catheter placement in the aneurysm was achieved by advancing a microcatheter over a microguidewire under biplane DSA road-mapping guidance. In a few cases, it was necessary to perform balloon angioplasty or infuse intraarterial papaverine for a proximal arterial vasospasm to advance a microcatheter into the aneurysm.
As over-the-wire angioplasty balloon catheters became commercially available, the angioplasty balloon would be advanced across the neck of the aneurysm whenever it was technically possible. This was done before selective catheter placement in the aneurysm, if it seemed that the balloon-assisted neck remodeling technique would be necessary. GDCs were then advanced and detached in the aneurysm under road-mapping guidance. During the 10 years of our experience, multiple technological advances in GDCs have occurred, and all of the currently available GDCs have been used at various times over that time. DSA series were frequently obtained during the course of the procedure to document the status of the aneurysm being treated and the adjacent normal vasculature. Repositioning of the catheter tip or exchange of the microcatheter for a different microcatheter was done when necessary. GDCs were advanced and detached in the aneurysm until the DSA series demonstrated that the aneurysm was occluded. If the aneurysm could not be completely occluded, additional GDCs were advanced to the point at which the placement of additional GDCs was no longer technically possible without jeopardizing the adjacent normal vasculature.
After detachment of the final GDC, the microcatheter was withdrawn and immediate postprocedural DSA series were obtained in the appropriate projection. Early in our experience, the anticoagulation was reversed with intravenous protamine, or it was allowed to reverse spontaneously before removal of the femoral arterial sheaths. During the later years of our experience, protamine was not administered, and the anticoagulation was allowed to reverse spontaneously before sheath removal, or femoral artery hemostasis was achieved by using the Perclose device (Abbott Laboratories, Redwood City, CA) for arteriotomy closure with full anticoagulation. Neither heparin nor any other anticoagulant or antiplatelet agent was routinely continued after the procedure, unless thrombus was apparent in the normal vasculature during the procedure. The patients were awakened from anesthesia and transferred to the neurosurgical intensive care unit. If the aneurysm was completely occluded at the end of the procedure, the patient’s systemic blood pressure was allowed to return to normotensive levels, or it was maintained at hypertensive levels (systolic pressure>150) if angiographic or clinical evidence suggested vasospasm. If the aneurysm was incompletely occluded at the end of the procedure, the systemic blood pressure was maintained at moderately low levels (systolic pressure <110) for 24 hours after the procedure.
Clinical Outcome
The severity of the SAH was clinically assessed at the time of admission by using the Hunt-Hess grading scale (22). Outcomes were assessed by using the Glasgow Outcome Scale (GOS) score (23).
Angiographic Outcome
Angiographic outcomes were characterized as complete occlusion, a dog-ear remnant, a residual neck, or a residual aneurysm (Figs 2–5) (24). Improvement of follow-up angiographic results, as compared with the immediate angiographic results, was defined as the improvement by one outcome category; for example, from a residual neck to a dog-ear remnant. A worse follow-up angiographic result was defined as worsening by one or more categories; for example, from a residual neck to a residual aneurysm.
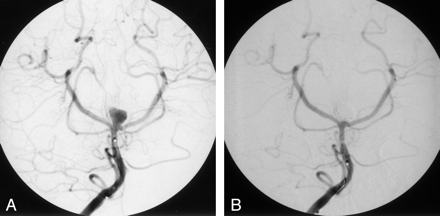
A 56-year-old man with Hunt-Hess grade 1 subarachnoid hemorrhage.
A, Towne-view right vertebral DSA demonstrates an 8-mm basilar caput aneurysm. The aneurysm was treated with seven GDCs measuring a total length of 51 cm.
B, Immediate post-treatment Towne-view right vertebral DSA demonstrates total occlusion of the aneurysm. Follow-up angiography obtained 6 months after treatment demonstrated persistent total occlusion of the aneurysm (not shown).
A 39-year-old man with Hunt-Hess grade 1 subarachnoid hemorrhage.
A, Towne-view left vertebral DSA demonstrates a 16-mm basilar caput aneurysm. The aneurysm was treated with seven GDCs measuring a total length of 180 cm. Immediate post-treatment DSA demonstrated a small “dog ear” neck remnant (not shown).
B, Towne-view left vertebral DSA obtained 6 weeks post treatment demonstrates slight enlargement of the “dog ear” neck remnant (arrow) at the right base of the aneurysm.
C, Towne-view left vertebral DSA obtained immediately after detachment of one GDC measuring 8 cm in the dog ear neck remnant demonstrates occlusion of the neck remnant and total occlusion of the aneurysm.
A 88-year-old man with Hunt-Hess grade 2 subarachnoid hemorrhage.
A, Lateral view right internal carotid DSA demonstrates a 3 × 6-mm anterior choroidal artery aneurysm. Oblique views (not shown) demonstrated direct origin of the anterior choroidal artery from the neck of the aneurysm. Significant atherosclerotic disease involving the cavernous right internal carotid artery and the right middle cerebral artery is also demonstrated.
B, Lateral view right internal carotid DSA immediately after detachment of four GDCs measuring a total length of 20 cm demonstrate occlusion of the fundus and dome of the aneurysm. A residual neck remnant (arrow) was intentionally left to preserve the origin of the anterior choroidal artery.
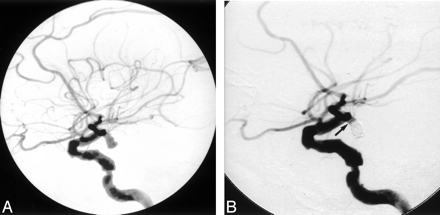
A 66-year-old woman with Hunt-Hess grade 3 subarachnoid hemorrhage.
A, Lateral view left internal carotid DSA demonstrates a 15-mm superior hypophyseal aneurysm. Seven GDCs measuring a total length of 140 cm were detached in the aneurysm. Immediate post-treatment DSA demonstrated persistent opacification of the aneurysm.
B, Six-month follow-up lateral view left internal carotid DSA demonstrates a residual aneurysm. The aneurysm was subsequently surgically clipped without complications.
Results
Patient data are shown in Table 1. The mean patient age at the time of initial treatment was 56.1 years. The mean clinical follow-up of patients who survived past the initial hospitalization was 19.1 months (range, 0.5–112 months). Angiographic follow-up was performed in 68 patients, who represented 93% of surviving patients. Although angiographic follow-up of at least 1 year was planned in most patients treated with GDCs, some patients did not return after the 6-month follow-up angiographic examination. The mean interval from initial treatment to longest angiographic follow-up was 11.6 months (range, 0.2–70 months). Of the aneurysms treated, 46 originated from the posterior circulation, whereas 37 originated from the anterior circulation (Table 2). The basilar caput (34 patients) and the anterior communicating artery complex (19 patients) were the most common locations for treated aneurysms, by a substantial margin. The mean aneurysm size was 7.8 mm in maximal diameter. Sixty-five aneurysms were smaller than 9 mm, 11 aneurysms were 10–14 mm, six aneurysms were 15–24 mm, and one aneurysm was 25 mm in maximal diameter. Forty patients had an associated daughter sac, while two patients had partial thrombosis of the aneurysm before the procedure, as demonstrated with angiography and cross-sectional imaging.
Patient data
Locations of aneurysms
The clinical outcomes at longest follow-up, as measured by using the GOS, are shown in Table 3. The mean GOS for all treated patients at longest follow-up was 4.2. Angiographic outcomes immediately after initial treatment and at longest angiographic follow-up are shown in Table 4. In 20 patients (29%) angiographic occlusion at longest follow-up was greater than it was immediately after initial treatment, whereas the degree of angiographic occlusion was worse in 10 patients (15%) (Fig 6). In 38 patients (56%), the angiographic result was unchanged at longest follow-up. Rates of incomplete occlusion, worsening of angiographic results at follow-up, and multiple endovascular treatments were similar among aneurysms at different locations.
Graph depicting degree of occlusion at follow-up angiography with respect to initial angiographic result.
Outcomes at longest clinical follow-up
Angiographic outcomes
No patient had rebleeding from the treated aneurysm during mean follow-up of 19.1 months after the initial coil procedure. Three patients required surgical repair following incomplete occlusion with GDCs. Two or more endovascular procedures involving GDC occlusion were required in 28 patients (34%); of these, five patients underwent three procedures, one patient underwent four procedures, and one patient underwent five procedures. No patient was treated with more than five stages of GDC occlusion. The frequency of patients requiring multiple endovascular treatments was uniform over the course of the study.
Complications related to the initial or subsequent endovascular procedures were identified in 16 patients (19%) (Table 5). In 14 patients, these complications resulted in no neurologic deficit or a temporary neurologic deficit, and they were considered minor. In four patients, complications related to femoral-artery vascular access or a reaction to the contrast agent occurred, and these were successfully treated. Four patients had transient neurologic symptoms related to thromboembolism during the procedure. In three patients, asymptomatic and non-occlusive vertebral dissection occurred, none of which caused hemodynamically significantly impaired blood flow or neurologic sequelae or required treatment. Two patients had coil migration or protrusion into the parent vessel; this was asymptomatic in one patient and caused a superior cerebellar infarct in another patient, who recovered completely. In one patient, perforation of a basilar tip aneurysm occurred early in the procedure. Although the patient did have a recurrent SAH, GDC treatment was continued, as it was believed to be the best and most immediate way to tamponade the hemorrhage. This recurrent hemorrhage complicated and lengthened the overall hospital course, but the patient completely recovered.
Complications of coiling
Major complications occurred in two patients (2%), resulting in serious neurologic disability in one patient and death in another patient. Both major complications were a result of thromboembolic stroke in the posterior circulation after endovascular GDC occlusion of aneurysms located at the basilar caput. Both patients received heparin during the procedure to maintain an ACT of twice the baseline value. Overall, when multiple procedures are accounted for, each GDC occlusion procedure was associated with a mortality rate of 0.8%, a permanent neurologic morbidity rate of 0.8%, and a temporary or non-neurologic morbidity rate of 11.6%. The rate of procedural complications did not substantially vary over time.
Discussion
Patient Selection
During initial clinical trials of GDCs for ruptured aneurysms and in the first few years of approved use that followed, endovascular treatment at our institution was reserved for patients in poor neurologic or medical condition who were believed to be poor surgical candidates. However, as experience has grown and practitioner skill improved, this trend has diminished, so that some patients now considered for endovascular GDC occlusion may be surgical candidates. For the overall series, the number of patients in poor clinical condition (those with Hunt-Hess IV or V lesions) at admission, and the mean admission Hunt-Hess grade were not significantly different from those of patients with SAH surgically treated at our institution over a similar time period (Friedman et al, unpublished data). However, an obvious bias exists in the selection of the location of ruptured aneurysms treated with GDC embolization: More than 60% of aneurysms in this series were located at the basilar caput or in the anterior communicating artery complex. A similar bias for basilar caput (3, 5, 11, 15) and anterior communicating artery (2) aneurysms has been reported in other endovascular series.
We limited our analysis to patients in whom one or more GDCs were actually detached, excluding patients in whom endovascular treatment was planned but aborted before detachment of the first GDC. Intent to treat data from Debrun et al (3) suggests that, in approximately 16% of patients in whom GDC occlusion of a cerebral aneurysm is planned, the procedure is aborted because the aneurysm is found to be poorly suited for GDC treatment. Although some additional morbidity may be associated with planned endovascular embolization procedures that are aborted before GDC detachment, this risk of morbidity is small in our experience. With recent improvements in technology, the percentage of aborted procedures has declined considerably in our experience. Furthermore, occasional endovascular exploration without the deposition of GDCs takes place at the time of initial diagnostic angiography; thus, an additional intervention or a delay in surgical treatment is not necessary.
Clinical Outcomes
The overall management mortality rate in this series of patients with acute SAH was 12%; all deaths occurred within 1.5 months of initial presentation. Although the mean GOS for the series overall was 4.2, nearly 70% of patients had a GOS of 5 at longest follow-up. The clinical outcomes largely reflect the initial SAH and its sequelae, and they are in keeping with the results from previous studies of the endovascular GDC occlusion of cerebral aneurysms (1–3, 5, 11,12, 15). Although the overall outcomes in our series also compare favorably to those of patients treated surgically after SAH, we can draw no conclusions regarding the relative effectiveness of the two procedures. Because of inherent selection factors, identified and unidentified, a retrospective comparison of the outcomes of endovascular and surgical treatments is unlikely to be valid.
Treatment-related complications were generally not associated with permanent neurologic deficit. Of the 16 complications of endovascular GDC treatment, 10 were associated with no neurologic deficit, and four were associated with temporary neurologic deficit without lasting disability. Thromboembolic complications occurred in six patients, resulting in serious neurologic morbidity in one patient and death in another patient. Our experience supports previous findings suggesting that thromboembolic events are infrequent but important sequelae of endovascular GDC occlusion of cerebral aneurysms (25–29). Both patients in our series who had serious morbidity or death had undergone GDC occlusion of a ruptured basilar caput aneurysm; in one patient, deterioration occurred 2 hours after the uncomplicated endovascular procedure, and emergent angiography showed unilateral P1 occlusion. No thrombolytic therapy was instituted, given the proximity of the recently ruptured basilar caput aneurysm. The patient had thalamic and midbrain infarctions and died 1 month later from related complications. The second patient had associated severe atherosclerotic basilar stenosis and did not wake up from anesthesia after a seemingly uncomplicated procedure. CT scans revealed infarction in the left occipital lobe, in the right parieto-occipital region, and in the right cerebellum. Subsequent angiography revealed no evidence of vasospasm, with preservation of the normal vasculature. Presumably, thromboemboli had traveled to the posterior circulation during the endovascular procedure. The patient remained in a persistent vegetative state and was transferred to a skilled nursing facility.
Of the two patients with temporary neurologic deficit, one patient had a single episode of homonymous hemianopsia, which was thought to be a transient ischemic phenomenon. The other patient had distal emboli to branches of the middle cerebral artery, which caused transient hemiparesis. Of the two patients with asymptomatic thromboembolic complications, one patient had occlusion of a P1 segment that spontaneously lysed before further intervention, whereas the other patient had a nonocclusive M1 segment thrombosis that lysed after the infusion of abciximab. Overall, 10 of 12 patients with complications not related to vascular access had aneurysms originating from the posterior circulation. Eight of these patients had aneurysms originating from the basilar caput; these patients include the one who died and the patient with a permanent neurologic deficit. This finding suggests an increased risk of complications with aneurysms originating from the posterior circulation. The occurrence of procedural complications was not associated with the size of the aneurysm.
Angiographic Outcomes
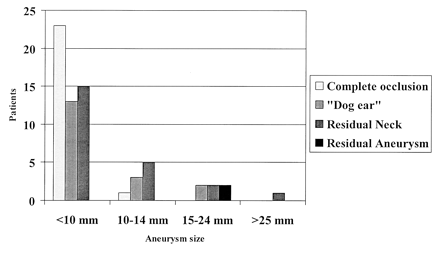
The 35% rate of complete occlusion and the 61% rate of a small remnant (dog ear or residual neck) at longest angiographic follow-up is generally consistent with findings in prior reports, when differences in grading and terminology are considered (1–5, 7, 11–13, 15) Aneurysms 15 mm or greater in maximal diameter were more likely to be incompletely occluded: Only two of seven aneurysms 15 mm or larger were completely occluded immediately after GDC treatment, with only one of seven completely occluded at longest angiographic follow-up (Fig 7). Partial aneurysm thrombosis was present in two aneurysms before treatment. In both cases, only partial aneurysm occlusion was achieved. The presence of a daughter sac from the aneurysm on angiograms was not relevant to the clinical or angiographic outcome or to the occurrence of procedural complications.
Graph depicting angiographic outcome at longest angiographic follow-up with respect to initial aneurysm size.
In four patients, residual aneurysm filling was present immediately after the initial treatment. One of these patients subsequently underwent surgical repair. In a second patient, follow-up angiography performed before planned additional endovascular GDC occlusion demonstrated increased aneurysm occlusion with a small residual neck, and no further treatment was offered. In the two remaining patients, their poor medical and neurologic condition precluded further treatment. In the absence of definitive data, we continue to advocate early surgical or additional endovascular treatment of ruptured aneurysms that demonstrate residual aneurysm filling after initial GDC treatment, whenever possible.
Fifty-six percent of treated aneurysms demonstrated no change in the degree of angiographic occlusion at follow-up (Fig 6). In particular, 74% of aneurysms that were completely occluded after initial GDC treatment remained completely occluded at angiographic follow-up; 44% of aneurysms changed by one or more angiographic category. Among these, the degree of aneurysm occlusion worsened in 15%. This finding underscores the need for angiographic follow-up in all patients, although the optimal duration and frequency of such follow-up remains uncertain: Our practice is to repeat angiography at 6 weeks, 6 months, and 1 year after initial treatment whenever possible, with additional follow-up as needed. Although worsening of the angiographic result was uncommon and not associated with adverse clinical sequelae, these data must be considered to reflect the short-to-intermediate term, based on the interval to angiographic follow-up. The long-term incidence of delayed recanalization or regrowth of aneurysms treated with endovascular GDC occlusion awaits further elucidation.
Rebleeding
The short-term prevention of rebleeding by using endovascular GDC occlusion for treatment of ruptured cerebral aneurysms was excellent. This result confirms the low incidence of rebleeding after GDC occlusion reported in earlier endovascular series, (3, 5, 10, 12, 14, 15, 24, 30, 31) and it also compares favorably to the results of surgical series (32–35). Furthermore, our data suggest that the degree of angiographic occlusion may not be an important factor in preventing rebleeding in the short term. This observation supports a benign natural history of aneurysm remnants after GDC occlusion, at least in the short term and as suggested by others (36, 37). Because incomplete occlusion with GDCs is most frequently a result of inadequate embolization of the neck or base of the aneurysm when the dome is successfully occluded, exclusion of the aneurysm dome from the intracranial circulation seems to be the key in preventing short-term rebleeding. It should be noted that three patients underwent surgical repair after incomplete coil placement; these patients were likely to have been at highest risk for rebleeding and selected for surgical treatment. Surgical repair of certain aneurysms incompletely treated with endovascular GDC occlusion is likely to remain an important clinical option for the foreseeable future. In addition, with new advancements of the technology, the percentage of completely occluded aneurysms after endovascular treatment is expected to increase in the future.
The favorable clinical results, low complication rate, and absence of rebleeding reported here represent the performance of a single endovascular surgeon over the course of 10 years. They reflect a consistent approach, skill set, and technique and therefore differ to some degree from the experience of centers with multiple endovascular practitioners with various stages of training and experience. The results can also be attributed to a conservative approach in patient selection and treatment; this is reflected in the low overall number of patients treated with GDC aneurysm occlusion at our busy center. Surprisingly, procedural complications and angiographic success did not vary significantly over the 10 years of the study, despite a seemingly higher ratio of more difficult aneurysms earlier in our experience. Although we were unable to define a learning curve in this single-practitioner series, this phenomenon may be steep but short in duration, as suggested by Debrun et al (3); thus, it may have been obscured over the course of a 10-year study. Despite the absence of obvious differences in outcomes over the time course of the study, it is important to recognize that the considerable evolution in both GDCs and treatment techniques over the 10 years limit the direct comparison of earlier cases to more recent ones.
Conclusion
Endovascular GDC occlusion of ruptured cerebral aneurysms has low morbidity and facilitates good overall treatment outcomes in patients who have had an aneurysmal SAH. Thromboembolic complications are the most important source of procedural morbidity after endovascular GDC occlusion; this risk underscores the need to develop strategies to minimize these occurrences. No patient treated with GDC occlusion had aneurysmal rebleeding during follow-up; this observation suggests excellent short-term effectiveness in preventing rebleeding. The durability of the treatment in preventing long-term aneurysmal rebleeding, as compared with that of surgical clipping, warrants further study.
Acknowledgments
The authors are indebted to Ms. Mary Soper for her assistance with manuscript preparation.
References
- Received February 1, 2002.
- Accepted after revision October 8, 2002.
- Accepted after revision October 8, 2002.
- Copyright © American Society of Neuroradiology