Abstract
BACKGROUND AND PURPOSE: Chronic abuse of toluene by inhalation causes variable white matter changes and thalamic hypointensity on T2-weighted MR images. The purpose of our study was to assess cranial MR findings in a large series of patients who chronically abuse toluene-containing solvents to investigate the factors causing the qualitative variability of white matter changes and thalamic hypointensity.
METHODS: We studied the neurologic signs, symptoms, and cranial MR findings in 41 patients who chronically abused thinner, a toluene-containing solvent. We classified white matter changes as diffuse or restricted. We tested the associations of the development of white matter lesions and thalamic hypointensity with patient age at onset of abuse and duration of abuse.
RESULTS: MR images revealed white matter lesions in 46% of the patients, atrophic dilatation of ventricles and sulci in 27%, and thalamic hypointensity in 20%. White matter changes were restricted in 53% and diffuse in 47%. The development of white matter changes and thalamic hypointensity were signficantly associated with duration of abuse longer than 4 years (P < .05 and P < .01, respectively).
CONCLUSION: White matter changes seem to start in the deep periventricular white matter, and they spread into peripheral white matter, causing the loss of gray matter-white matter differentiation with continued toluene abuse. The deposition of iron due to demyelination and axonal loss is the most probable mechanism for the thalamic hypointensity found in solvent abusers.
Organic solvent inhalation is a common form of substance abuse in children and young adults (1, 2). The low cost and easy availability of organic solvents have led to increases in the number of young abusers in many countries. Toluene is the major component of organic industrial solvents that is thought to cause the neurotoxicity seen in solvent abusers (3, 4).
Demyelination and gliosis in the cerebral and cerebellar white matter are the histologic changes reported in chronic toluene abusers (5, 6). Cerebral and cerebellar atrophy, multifocal or diffuse white matter changes, and loss of demarcation between cortex and white matter are cranial MR imaging findings in chronic toluene encephalopathy (6–9). White matter lesions in chronic solvent encephalopathy were classified as restricted, diffuse, or intermediate, according to the extent of signal intensity changes (8) revealed by MR imaging. Some have speculated that the possible variability in abused substances, the duration of abuse, and the quantity and methods of abuse might be factors in the qualitative differences in the white matter changes (8).
Symmetric hypointensity in the thalami and basal ganglia on T2-weighted cranial MR images is also reported in chronic solvent abuse (8, 10, 11). Some investigators have suggested that iron deposition and the partition of toluene into the lipids of cell membranes explain this finding (8, 11).
We studied the neurologic symptoms and signs and the cranial MR findings in a series of patients who chronically abused solvents (ie, thinner). The purpose of our study was to investigate the associations of the development of white matter changes and thalamic hypointensity on T2-weighted images with patient age at onset of abuse and duration of solvent abuse.
Methods
Patient Characteristics
We examined 41 patients who chronically abused solvents (40 male, one female; age range, 14–23 years; mean age, 17.5 years). All patients were homeless people who were admitted for a solvent-abuse rehabilitation program. In all patients, we recorded information regarding the abused substance, duration of abuse, method of abuse, and amount of substance consumed daily. Liquid thinner, an organic solvent used in painting, was the substance abused by all of our patients. Patients dependent on other drugs or alcohol were excluded from the study. Mean patient age at the beginning of abuse was 12.9 years (range, 9–19 years). Mean duration of abuse was 4.6 years (range, 1–11 years). The common method of abuse was sniffing or huffing (ie, inhalation by mouth) by using a rag soaked with liquid thinner. Most patients stated that their daily thinner consumption was highly variable, because they did not have a regular income with which to buy thinner. The information obtained from all patients revealed that the amount of daily thinner consumption had increased with time in the first 2 years of abuse and reached a plateau in the 2nd or 3rd year of abuse. We deemed calculation of daily dosage of inhaled impossible because of the easy vaporization of thinner and incalculable issues related to method of abuse (eg, variability in the frequency of huffing, the amount of thinner inhaled during each huffing episode, different physical properties of the rags used).
All patients underwent a detailed neurologic examination before undergoing cranial MR imaging. We assessed patients for the presence of any metabolic, infectious, or inflammatory neurologic disease by review of detailed clinical history and findings of routine laboratory and cranial MR examinations. One patient with a history of epilepsy before onset of abuse was excluded from the study.
MR Imaging
After a period of abstinence of 4 to 7 days, all patients underwent cranial MR examination on a system equipped with a 1.0-T superconducting magnet (Magnetom; Siemens, Erlangen, Germany). Examinations included sagittal and axial T1-weighted spin-echo sequences (600/15/1, TR/TE/NEX) with 5.0-mm section thickness, axial T2-weighted spin-echo sequences (2620-3100/17-85/2) with 5.0-mm section thickness, and coronal T2-weighted fast spin-echo sequences (4800/100/2) with 5-mm section thickness. With all sequences, a 192–256 × 200–256 matrix and a 180–225 × 200–225-mm field of view were used. Two neuroradiologists (K.A., S.S.) independently interpreted images.
White matter changes on proton density-weighted and T2-weighted images were defined as diffuse or restricted, similar to the classification Yamanouchi et al (8) described. Diffuse changes were widespread, extending to subcortical white matter, with focal or diffuse loss of gray matter-white matter differentiation. Restricted changes were periventricular white matter alterations without a loss of gray matter-white matter differentiation. Unlike Yamonouchi et al, we used the diffuse classification for both the diffuse and intermediate types they described. Because gray matter-white matter differentiation was preserved in all of our cases, we did not have a criterion with which to subclassify the white matter changes according to the extent of loss of gray matter-white matter differentiation, as Yamonouchi et al did. We examined the corpus callosum by using the method Weis et al (12) described.
Statistical Analysis
We assigned patients into two groups according to the duration of their abuse: those who abused the substance for less than 4 years and those who did so for more than 4 years, because 4 years was the mean duration of abuse. We also grouped patients according to age at which abuse began: those who started when they were younger than 12 years and those who started when they were older than 12 years, because 12 years was the mean age at onset of abuse. We tested the association of the development of white matter changes with duration of abuse and with age at onset of abuse. We also compared differences between those with and those without thalamic hypointensity relative to duration of abuse. The Fisher exact test was used for statistical analyses. A P value of less than .05 was selected to indicate a statistically significant difference.
Results
Findings are summarized in the Table.
Distribution of clinical and MR imaging findings with respect to the duration of toluene abuse
Clinical Findings
Of 41 patients, 34 (83%) reported insomnia and forgetfulness; 30 (73%), anosmia; 22 (54%), tremors of the extremities; and 15 (37%), tinnitus. One patient had had recent episodes of generalized convulsions.
Neurologic examination revealed ataxia and disturbed performance on cerebellar testing in 20 patients (49%), tremors of the extremities in 15 (37%), hyperactive tendon reflexes in 11 (27%), rigidity in 10 (24%), dysarthria in four (10%), and nystagmus in two (5%).
MR Imaging Findings
Cranial T2-weighted MR images in 19 patients (46%) revealed high-signal-intensity white matter changes. Patients with findings of abnormal white matter changes had a mean duration of abuse of 6.8 years (range, 3–11 years). The development of white matter changes on T2-weighted images was significantly associated with duration of abuse longer than 4 years (P < .05). However, white matter changes were not significantly associated with age at onset of abuse (P > .05).
Periventricular white matter and the centrum semiovale were the most common locations for white matter changes in 19 (46%) of 41 patients (Fig 1). T2-weighted images also showed increased signal intensity in the cerebellar white matter (15 patients [37%]), internal capsule (13 patients [32%]), and brain stem and upper cervical cord (11 patients [27%]) (Fig 2A and B). T1-weighted and T2-weighted images showed atrophic dilatation of ventricles and cerebral and cerebellar sulci in 11 patients (27%). Thinning of the corpus callosum was revealed in nine patients (22%), whereas no signal intensity change was found in the corpus callosum (Fig 2C and D). T2-weighted images revealed symmetric hypointensity in the thalami in eight patients (20%), hypointense red nuclei and substantia nigra in two, and hyperintense dentate nuclei in one (Fig 3). On T2-weighted images in three patients, small, round, nonspecific hyperintense lesions were present in the cerebral white matter; these lesions were similar to those of age-related white matter changes. Coronal T2-weighted images in the patient who had new-onset epilepsy revealed increased signal intensity and atrophy in the left hippocampus. White matter changes on T2-weighted images were classified as diffuse in nine (47%) of 19 patients who had abnormal MR imaging findings (Fig 3D). The mean duration of abuse of patients with diffuse white matter changes was 7.9 years (range, 6–11 years). All patients with diffuse white matter changes had findings of cerebral-cerebellar atrophy and thinning of the corpus callosum.
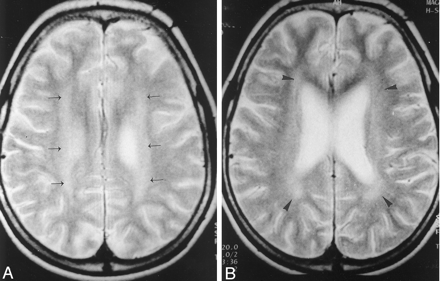
Axial T2-weighted (2900/85/2 [TR/TE/NEX]) images in a 16-year-old patient who had inhaled toluene for 6 years.
A, High signal intensity is seen in the centrum semiovale (arrows) on both sides. The peripheral cerebral white matter and gray matter-white matter differentiation are preserved.
B, High-signal-intensity changes involve the frontal and parietal periventricular white matter (arrowheads). The pattern of white matter changes is compatible with that of the restricted type. Note that the lateral ventricles and cerebral sulci are enlarged.
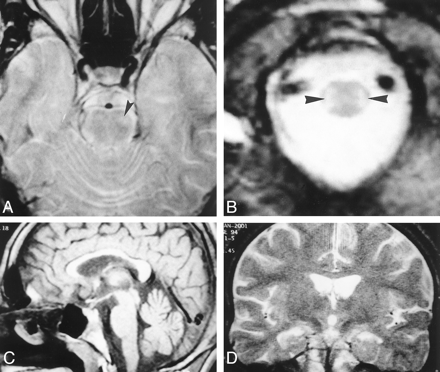
MR images in a 19-year-old patient who had abused toluene for 8 years.
A, Axial T2-weighted (2620/85/2 [TR/TE/NEX]) image at the level of the brain stem shows a hyperintense lesion in the anterolateral part of pons (arrowhead). Note that gray matter-white matter differentiation is lost in the anterior part of the left temporal lobe.
B, Axial T2-weighted (2620/85/2) image at the level of lower medulla oblongata shows symmetric hyperintensity along the spinocerebellar tracts (arrowheads).
C, Sagittal T1-weighted (600/15/1) midline image shows thinning of the corpus callosum, which is more prominent in the body and genu. Note the enlarged cerebellar sulci.
D, Coronal T2-weighted image (4800/100/2) reveals a lack of signal intensity abnormality in the corpus callosum.
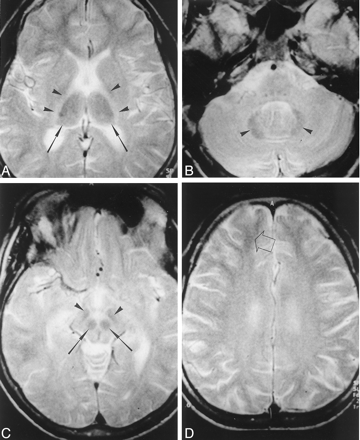
Axial T2-weighted MR images (3000/80/2 [TR/TE/NEX]) in a 22-year-old patient who had abused thinner for 11 years.
A, Symmetric hypointensity is present in the thalami (arrows). Symmetric hyperintensity exists in the posterior limbs of the internal capsule (arrowheads).
B, At the level of the posterior fossa, generalized increased signal intensity in the cerebellar white matter emphasizes the dentate nucleus (arrowheads).
C, At the level of midbrain, red nuclei (arrows) and substantia nigra (arrowheads) are hypointense.
D, Diffuse high-signal-intensity in the centrum semiovale causes loss of gray matter-white matter differentiation. Gray matter-white matter differentiation is preserved only in the subcortical-frontal white matter (arrow). The cerebral sulci are mildly dilated.
T2-weighted images showed restricted white matter changes in 10 (53%) of 19 patients with abnormal MR imaging findings. The most common restricted white matter changes revealed by T2-weighted imaging were patchy, high signal intensity in parietal areas, frontal periventricular areas, or both (Fig 4). The mean duration of abuse of patients with restricted white matter changes was 5.8 years (range, 3–8 years).
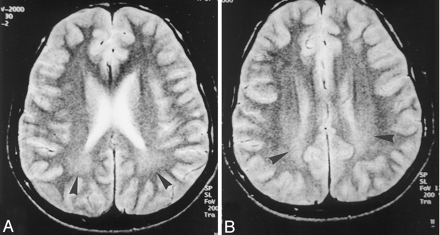
Axial T2-weighted (3000/80/2 [TR/TE/NEX]) images in a 16-year-old patient who had inhaled thinner for 3 years.
A and B, Bilateral, symmetric hyperintensity is present in the parietal periventricular white matter (arrowheads). Note that gray matter-white matter differentiation is preserved. The widths of the cerebral sulci are normal. A suggests that the hyperintense findings may be caused by terminal myelination, which is a normal MR imaging finding in children. However, B shows that the lesions extend to the bilateral centrum semiovale, indicating that hyperintense findings were true lesions rather than terminal myelination.
All patients with abnormal white matter changes on cranial MR images had neurologic symptoms and signs. Only those patients with diffuse white matter changes had nystagmus and dysarthria. Patients with diffuse white matter changes and those with restricted changes did not differ with respect to the presence of other neurologic symptoms and signs.
The mean duration of abuse in patients with thalamic hypointensity on T2-weighted images was 8.1 years (range, 6–11 years). No patient with thalamic hypointensity had abused thinner for less than 5 years. The significant difference between the patients with and those without thalamic hypointensity was related to duration of abuse (P < .01). T2-weighted images in six (75%) patients with thalamic hypointensity showed diffuse white matter changes, and those in the remaining two patients (25%) showed the restricted pattern. All patients with thalamic hypointensity had cerebral-cerebellar atrophy.
Discussion
In 1951, Clinger et al (13) reported the first cases of volatile substance abuse by inhalation. In their report, the abused substance was gasoline. Today, organic solvents contained in industrial and domestic products are the most commonly abused volatile substances. The abuse of volatile substances is still a social and health problem in many countries.
Toluene, or methylbenzene, is a lipid-soluble aromatic hydrocarbon that is commonly used as an organic solvent in many industrial and domestic products such as thinners, glues, and spray paints. Toluene is the main component of organic solvents and is blamed for the acute and chronic toxic neurologic effects of solvent inhalation.
Inhaled toluene is rapidly absorbed by the lungs, from where it easily enters the highly vascularized lipid-rich brain. Because of its high lipid solubility, toluene accumulates in lipid-rich tissues such as brain. Toluene is degraded into benzoic acid by oxidation and is conjugated with glycine to form hippuric acid in the liver. The kidneys excrete hippuric acid.
Long-term inhalation of toluene-containing solvents causes irreversible central nervous system damage. Toluene-induced chronic toxic encephalopathy causes cerebellar symptoms and signs such as ataxia, tremors, and nystagmus (14–17). The associations of chronic toluene inhalation with cognitive impairment, psychiatric disorders, spasticity, and Parkinson disease have been reported (18–24).
Byrne et al (19) reported an association between chronic toluene inhalation and temporal lobe epilepsy. They reported an incidence of 13.6% for temporal lobe epilepsy among chronic solvent abusers. Only one of our patients (2%) had epilepsy and findings of mesial temporal sclerosis at cranial examination.
The significant association of white matter changes on cranial MR images with the degree of cognitive impairment has been reported (25). In our study, we observed concordance between the presence of white matter changes on cranial MR images and the neurologic symptoms and signs; all patients with white matter changes had neurologic deficit. The association of white matter changes with abuse longer than 4 years suggests that white matter lesions are the result of a cumulative toxic effect of inhaled toluene.
Xiong et al (7) and Yamanouchi et al (8) described white matter changes in the centrum semiovale, posterior limb of the internal capsule, the ventral part of pons, the cerebellar peduncles, and the cerebellar white matter on cranial MR images in patients who chronically abuse toluene. Demyelination and gliosis are the histopathologic changes underlying these white matter lesions on cranial MR images (5, 6). Toluene inhalation causes activation and morphologic changes of astrocytes in the rat brain, especially in the cerebellum and hippocampus (26, 27). White matter changes are multifocal in the restricted type, but alterations in the diffuse type are widespread, causing loss of gray matter-white matter differentiation. Yamanouchi et al (8) speculated that the abuse of different toluene-containing substances might cause different patterns of white matter changes. However, all of our patients abused the same substance. Also, no significant difference between the age at the beginning of the abuse and the method of inhalation was present to explain the difference in the types of white matter changes. Yamanouchi et al (8) also suggested that restricted white matter changes might be an early stage of diffuse white matter changes. The results of our study support this suggestion. Our patients with diffuse white matter changes had abused the substance longer than had patients with restricted changes. The focal preservation of gray matter-white matter differentiation in the subcortical white matter in our patients with diffuse changes indicates that white matter changes start in the periventricular area and then extend into the subcortical white matter. Correspondingly, Rosenberg et al (6) reported the histologic findings in a case in which white matter changes were more prominent in the periventricular area than in the subcortical white matter. Also, only 20% of our patients with restricted changes had findings of atrophy on MR images, although all patients with diffuse white matter changes had cerebral atrophy. The presence of nystagmus and dysarthria in patients with diffuse white matter changes seems to have resulted from extensive demyelination in the brain stem and cerebellum. Therefore, white matter changes in cases of chronic toluene inhalation seem to start in the periventricular area, where they create a restricted pattern. These changes may transform into the diffuse type, together with the development of cerebral atrophy, if the duration of abuse is long enough.
T2-weighted hypointensity in the thalami and basal ganglia is a well-known MR finding. Kamran and Bakshi (28) reported hypointensity in motor and visual cortices. We did not identify cortical hypointensity in any T2-weighted images. Controversial reports about the nature of the thalamic hypointensity exist. Xiong et al (7) reported three cases of chronic toluene encephalopathy and discussed the nature of the thalamic hypointensity. They suggested that the accumulation of iron might cause thalamic hypointensity.
Unger et al (11) studied eight cases of chronic toluene encephalopathy. Histologic findings at autopsy in one of their patients who died from a toluene overdose did not show thalamic iron deposition. However, cranial MR examination had not been performed in the patient. Therefore, pathologic-radiologic correlation was not possible. Unger et al also designed an experimental model to determine the source of thalamic hypointensity. They proposed that the partition of lipid-soluble toluene into brain lipids might cause the thalamic hypointensity. The hypothesis suggested by Unger et al cannot explain the absence of thalamic hypointensity in our patients who abused toluene for less than 5 years. If the partition of toluene into brain lipids were the only cause, thalamic hypointensity would be demonstrated in all patients, including those who had abused the substance for less than 4 years. The significant association between the development of thalamic hypointensity and the duration of abuse suggests that thalamic hypointensity develops as a result of a chronic abuse.
The results of the experimental study conducted by Unger et al demonstrated the correlation of the degree of hypointensity on T2-weighted images with the concentration of toluene. We could not calculate the daily dosage of inhaled toluene and thereby test its association with the development of thalamic hypointensity, which was a limitation of our study. The partition of toluene into brain lipids might cause thalamic hypointensity after a limiting dosage is reached. Contrary to this hypothesis, T2-weighted images in 51% of our patients did not reveal thalamic hypointensity, although the daily thinner consumption in our patients reached the plateau in their 3rd year of abuse.
Demyelination, dysmyelination, infarction, and neurodegenerative diseases are reported to cause extrapyramidal iron deposition and hypointensity in the thalami, basal ganglia, and red nuclei on T2-weighted images (29–31). Some have suggested that disruption of the physiologic axonal transport of iron might result in the accumulation of iron in the thalami and basal ganglia. With a similar mechanism, demyelination and axonal loss induced by the chronic inhalation of toluene may reach a level sufficient to cause iron deposition and thalamic hypointensity on T2-weighted images.
Conclusion
Chronic toluene abuse results in the development of hyperintense white matter lesions and thalamic hypointensity on T2-weighted images, which is associated with the duration of abuse. Although the partition of toluene into brain lipids may theoretically contribute to the development of thalamic hypointensity, it does not seem to be the major factor. The deposition of iron caused by demyelination and axonal loss seems to be the most probable mechanism for thalamic hypointensity on T2-weighted images in toluene-containing solvent abuse.
References
- Received January 3, 2002.
- Accepted after revision April 2, 2002.
- Copyright © American Society of Neuroradiology