Abstract
Summary: Granular cell tumor (GCT) is an infrequent benign neoplasm, which Abrikosoff accurately described in 1926. GCT probably has a neural crest cell origin. We present the clinical and imaging findings in a 45-year-old woman with a GCT involving the palate. CT and MR imaging revealed perineural tumor spread along the greater and lesser palatine nerves. We emphasize the peculiarity of the palatine location and the perineural spread of GCTs.
Granular cell tumor (GCT) is an uncommon benign lesion affecting the mucous membranes of the upper aerodigestive tract. About one third of all GCTs occur in the head and neck region (1). The most common site is the anterior part of the tongue. Most GCTs are benign, but approximately 10% have malignant behavior (2). Metastases to the regional lymph nodes and distant metastases are observed; however, to our knowledge, perineural infiltration has never been described.
First considered as a myoblastic tumor, GCT is now believed to be of primitive neuroectodermal origin (3, 4). This tumor arises predominately from the submucosa and usually presents as a small, slowly enlarging lesion; it has been reported to cause severe pain (4). Diagnosis is often delayed.
We report a case of painful GCT of the palate, with perineural spread along the maxillary division (V2) of the trigeminal nerve in a 45-year-old woman. Herein, we discuss its clinical, radiologic. and histologic aspects. The initial symptom was progressive, severe pain. Extensive clinical and imaging examinations were unsuccessful in revealing any tumor during a 3-year period.
Case Report
A 45-year-old woman had a 2-year history of constant left-sided palatine pain between the first molar and the midline that was exacerbated by contact with food or hot beverages and by deep inspiration. The patient’s medical history included an excision of a cutaneous melanoma in the region of the buttock 1 year before. Physical examination revealed no abnormality other than the stigmata of an extraction of tooth 26 performed for pain relief. Findings from initial CT and MR imaging examinations, which were focused on the pathway of the trigeminal nerve, were considered normal. Treatment with antibiotics, steroids, antalgics (carbamazepine, Tegretol), and opiates was unsuccessful. The patient was referred for a new evaluation 1 year later. At that time, oral examination demonstrated a subtle swelling of the anterior left palate. MR imaging showed a 1-cm submucosal left palatine lesion that was isointense on T1-weighted spin-echo (SE) images and slightly hyperintense on T2-weighted SE images, relative to muscle (Fig 1). Similar to the normal palatine submucosa, this lesion was enhancing homogeneously after the IV administration of gadolinium-based contrast agent, Surgical exploration did not reveal any resectable tumor, and large biopsy was performed with histopathologic examination. The latter showed only malpighian metaplasia without any evidence of tumor.
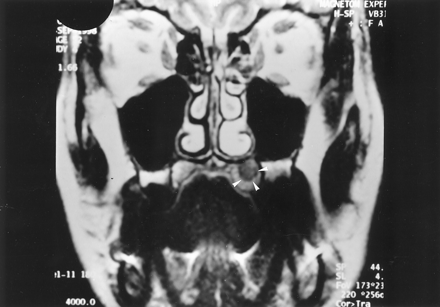
Coronal T2-weighted (TR/TE/NEX, 4000/99/4) MR image 1 year after initial presentation shows an oval, left palatine lesion (arrowheads) that is hyperintense relative to muscle.
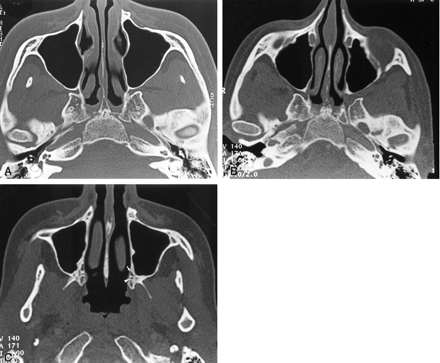
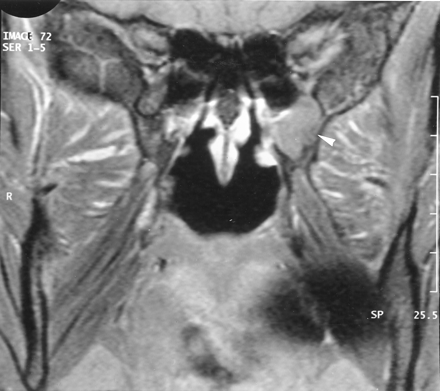
The recurrence of pain 2 months after biopsy prompted further imaging evaluation. CT demonstrated enlargement of the left pterygopalatine fossa (Fig 2B), which was moderately enhancing on contrast-enhanced CT scans. Comparison with the previous imaging examinations confirmed that the presurgical imaging findings were normal (Fig 2A). The pterygopalatine mass appeared isolated, without any enlargement or demineralization of the lesser and greater palatine foramina (Fig 2C).
Axial bone-window CT scans.
A and B, Images obtained through the pterygopalatine fossa at initial presentation (A) and 14 months later (B) show widening of the left pterygopalatine fossa.
C, Image obtained through the palatine foramen at the same time as the image in B shows no abnormality of the left greater (arrow) and lesser (arrowhead) palatine foramina.
Moreover, MR imaging demonstrated a palatine lesion with a location and signal intensity similar to those of the lesion described 1 year before (Fig 3). Contrast-enhanced T1-weighted fat-suppressed images demonstrated obliteration of the soft tissue of the pterygopalatine fossa, with enlargement of the foramen rotundum and without any detectable involvement of the cavernous sinus on the same side (Fig 4). This infiltration had the same signal intensity features as those of the palatine tumor; namely, isointensity on T1-weighted SE images and hyperintensity on T2-weighted SE images relative to muscle. This infiltration was moderately enhancing after the IV administration of gadolinium-based contrast material. Surgical resection of the bone palate was performed without exploration of the pterygopalatine fossa, because the findings of perioperative histopathologic examination failed to establish the diagnosis of malignancy. The frozen sections of the palatine specimen revealed only a fibrosclerotic reaction without any evidence of tumor. A second histopathologic study of the palatine section, including S-100 immunohistochemical staining, revealed a GCT; this finding was based on diffuse proliferation of large oval cells with eosinophilic and granular cytoplasm (Fig 5).
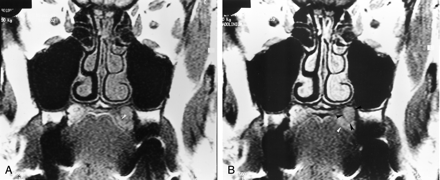
Coronal T1-weighted (550/15/4) MR images obtained at the same time.
A, Image obtained 14 months after the onset of symptoms shows a left subtle submucosal palatine mass that is isointense to muscle. Abnormal intermediate signal intensity of the surrounding bone (arrowhead) is depicted.
B, Image shows a discrete enhancement of the left palatine lesion (black arrowheads). Its margins are better delineated on this image. Intermediate signal intensity of the surrounding bone (white arrowhead) is depicted.
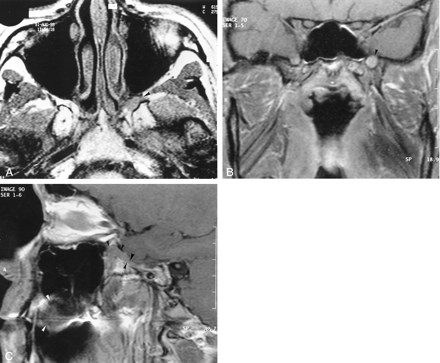
T1-weighted MR images.
A, Axial image (520/14/4) obtained 14 months after the onset of symptoms shows loss of the normal fat signal hyperintensity in the left pterygopalatine fossa (arrowhead). This finding represents infiltration of the latter by a lesion that is isointense relative to muscle.
B, Coronal enhanced image (520/14/3) obtained through the base of the skull. The left foramen rotundum is enlarged and infiltrated by a discrete, hyperintense, soft-tissue mass (arrowhead).
C, Sagittal enhanced image (520/14/3) obtained through the left pterygopalatine fossa shows the palate lesion (white arrowheads), which extends through a widened foramen rotundum into the pterygopalatine fossa (black arrowheads).
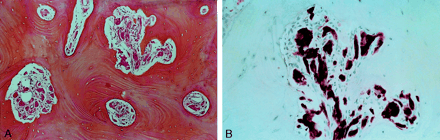
Photomicrographs (magnification ×100).
A, Well-organized clusters of eosinophilic granular cells (arrowhead) are depicted within haversian bone (hematein phloxine safranin).
B, At a higher magnification, the granular cells are distributed in small clusters. The cells have an elongated or spindle shape, with abundant cytoplasm and small basophilic nuclei (arrowhead). No high-grade nuclear atypia, necrosis, or mitoses are present (hematoxylin-eosin with S-100 protein).
After a brief improvement in the symptoms, severe unrelenting pain reappeared. Because repeat MR imaging showed an enlarging mass of the pterygopalatine fossa (Fig 6), large maxillectomy was performed. Perioperative histopathological examination showed recurrence of the tumor on the palate without any evidence of further perineural spread. Pathologic evaluation after deossification showed extensive perineural spread along the palatine nerves without free neural margins.
Enhanced fat-saturated coronal T1-weighted MR image (520/14/3) obtained 6 months after the image in (Figure 3 shows enlargement of the lesion of the left pterygopalatine fossa arrowhead.
Discussion
Abrikosoff first described GCT as granular cell myoblastoma in 1926, because the cells found in this tumor were thought to be derived from myoblasts (1). An ultrastructural study by Thompson in 1984 revealed that the GCTs were in direct continuity with striated muscle (1). However the histiocytic origin of GCT, as proposed by Whitten (5), was strongly supported by the finding of autophagocytic vacuoles and positive immunohistochemical staining for α-1 antitrypsin in some tumors (1, 3). Since then, other investigators (3) have proposed a neurogenic origin on the basis of the close association of the tumor with the nerves and ultrastructural findings of neurofilaments in the granular tumor cells. This theory was supported by Holland et al (6), who demonstrated S-100 staining in Schwann cells but not in myofibers. The S-100 protein is found in neurons and in Schwann cells in the late phase of cell development. GCT has been found throughout the body. About 50% occur in the head and neck, especially the tongue, which is the most common site and accounts for approximately 30% of lesions (1, 4, 7–11). Other involved areas are the skin and subcutaneous tissues (30%), the breast (15%), the respiratory (10%) or gastrointestinal tracts, and the biliary system (4, 10). To our knowledge, only one case report (9) describes GCT of the palate.
Most studies demonstrate a female predilection, and more than half of the cases have occurred in African American patients (3, 4, 6, 10). The age range varies from 4 months to 89 years, with a mean age between the fourth and sixth decades of life (2, 10, 11). Two distinct subtypes have been described: 1) a congenital epulis, or a gingival GCT of infancy, and 2) a more common adult GCT (3, 10, 12, 13).
The tumor typically appears as a solitary lesion, although multifocal tumors at the first presentation are reported in 4–10% of cases (3, 7–9, 11–13). The histologic diagnosis of GCT is based on the presence of polygonal cells with eosinophilic granular cytoplasm and small nuclei. Positive staining with S-100 protein is strong evidence for this tumor (1, 3, 7–10).
The histologic diagnosis may be difficult because pseudoepitheliomatous hyperplasia that overlies the GCT may lead to its confusion with squamous cell carcinoma (1, 6). Most GCTs are benign, but malignancy is described in approximately 10% of cases (3, 7, 8). The malignant potential of a GCT is suggested by cellular and nuclear pleomorphism, necrosis, wide cellular sheets, and mitotic activity. Tumors with such features have an uncertain malignant potential and require close follow-up (8). In cases of a metastatic lesion with extensive infiltration of the adjacent tissues and enlargement of the lymph nodes that is associated with the histologic features described earlier, the diagnosis of malignancy is highly suggested (8, 10).
The more common sites of metastasis are the regional lymph nodes, bone, peripheral nerves, peritoneal cavity, and lung (1, 8). In our case, the perineural spread of the palatine tumor into the foramen rotundum and the pterygopalatine fossa proves the potential malignant behavior of the tumor, despite its benign appearance at histopathologic evaluation. Perineural extension of head and neck tumors is a well-known form of tumor spread. Squamous cell carcinomas and adenoid cystic carcinoma are the most common primary tumors to spread via a perineural mechanism (14–16). In most of cases, the tumor spreads directly in the perineural or the endoneural tissue planes along the path of least resistance. Spread through perineural lymphatic channels may explain how the tumor may spread discontinuously with so-called skip areas (14, 15). This phenomenon can occur relatively early, and the tumor may extend a considerable distance without any involvement of the adjacent structures or lymph nodes. Clinical evidence of perineural tumor infiltration may present as a burning or stinging pain and hypesthesia in the distribution of a trigeminal nerve. Sometimes, neoplasms may exist in the nerves for many years without producing any symptoms. The second and third divisions of the trigeminal nerve and the facial nerve were the most common nerves to be involved by perineural infiltration. Recognition of this mode of spread is of great importance, because the treatment and prognosis are altered when perineural extension occurs. The diagnosis is suggested by CT and on MR imaging findings. Nonenhanced and contrast-enhanced MR fat-suppressed images allow the identification of perineural spread. Imaging features include enlargement and/or enhancement of a nerve or widening of the foramina and canals through which the nerves pass (14–16). Therefore, repeat imaging studies with careful examination of the pathway of the trigeminal nerve (eg, palatine foramina, pterygopalatine fossa, foramen rotundum, cavernous sinus, Meckel cave) should be performed in patients who present with severe pain, with pain in the same location, or pain that is resistant to major antalgic treatment. Perineural infiltration has been recently reported in some benign lesions such as granulomatosis, meningiomas, and inflammatory pseudotumor (17).
Few reports of the imaging findings of GCT exist because these tumors usually measure less than 3 cm in diameter at the time of diagnosis (4). Negative findings at initial imaging examination did not rule out the diagnosis of a tumor, especially when the lesion is small and has a signal intensity similar to that of the adjacent tissues. One report of GCT of the subcutis of the trunk described the lesion as an inhomogeneous subcutaneous mass that was isointense on T1-weighted images and hypointense on T2-weighted images, relative to the adjacent muscle. The low signal intensity of GCTs on T2-weighted imaging may be due to their abundant interstitial collagen fibers and smaller amount of cellular components; these findings are characteristics of this tumor (4). Other reports described GCTs as being slightly hypointense on T1-weighted MR images. On T2-weighted MR images, these lesions show heterogeneously increased signal intensity (10, 11). The degree of contrast enhancement has been reported in two cases, which had homogeneous contrast enhancement (10, 11).
Surgical excision is the treatment of choice of GCTs. In most cases, this procedure is curative despite incomplete excision of the lesion (1–3, 8–10, 13). However, a small percentage of recurrence is described. The recurrence rate after adequate local excision is 8%, and that with a positive excision margin is 21–50% (1, 3, 10, 11). Regional lymph node dissection is recommended when tumors have rapid growth or when they exceed 4 cm in diameter (8). Radiation therapy and chemotherapy have been used in malignant GCTs. Their effectiveness remains unproven (3, 8).
Conclusion
GCT is rarely located within the submucosa of the palate. The small size of this lesion at the onset of symptoms makes the radiologic diagnosis difficult and leads to repeat imaging examinations in patients referred because of chronic and severe pain. Even histologic study may fail to prove diagnosis because the lesion lies deeply and may be surrounded by an important malpighian hyperplasia. A discontinuous perineural infiltration with skip areas is revealed in this small tumor, with histologically benign features.
Acknowledgments
We gratefully acknowledge the assistance of D. Rocher with the artwork and W. Grauer, MD, in the preparation of this manuscript.
References
- Received July 3, 2001.
- Accepted after revision December 27, 2001.
- Copyright © American Society of Neuroradiology