Abstract
BACKGROUND AND PURPOSE: We previously reported that pretreatment with external ultraviolet (UV) irradiation at 325 nm before blood exposure prevented the development of chronic vasospasm in rabbit common carotid arteries. The purpose of this study was to investigate the preventive effect of endovascular UV light on vasospasm after blood immersion by using the same animal model.
METHODS: The right common carotid arteries in 63 rabbits were enclosed in silicon cuffs on day 0. Sheaths were empty or filled with clotted blood. Thirty minutes after the placement of the sheaths, either alone or with blood, the common carotid arteries were subjected to UV or visible light (442 nm) irradiation via an endovascular approach at a fluence rate of 0.17 W/cm2. The animals were killed on day 2, 9, or 30. Digital subtraction angiography was performed on days 0 and 2 and at the end point to evaluate the degree of vasospasm.
RESULTS: UV treatment significantly prevented the development of vasospasm on day 2. On days 9 and 30, there were no significant differences between UV-treated animals and control animals. The preventive effect reached an approximate plateau with an irradiation time of 10 s. No severe vascular injury, such as perforation, occurred in response to UV treatment during the observation period. UV light was significantly more effective than visible light in preventing vasospasm (P < .001).
CONCLUSION: These results suggest that endovascular UV irradiation after blood exposure has a prophylactic effect on vasospasm and suggest a dependence on irradiation wavelength and duration of irradiation.
Chronic cerebral vasospasm is the major cause of morbidity and mortality in patients after aneurysmal subarachnoid hemorrhage. In approximately half of the cases, delayed neurologic ischemic deficits occur. Despite therapy, 15% to 20% of patients suffer stroke or death as a result of vasospasm (1). Although the pathogenesis of cerebral vasospasm is not yet understood despite intensive investigations, inflammation has long been suspected to play an important role in the development of the process after subarachnoid hemorrhage (2). Several lines of evidence seem to support the theory of inflammation. These include the presence of inflammatory cells in the walls of vasospastic vessels (3), elevated levels of several inflammatory mediators in the CSF of the patients after subarachnoid hemorrhage (4, 5), higher systemic temperature in patients with severe vasospasm (6), and the prevention of experimental vasospasm with anti-inflammatory drugs (7), immunosuppressants (8, 9), and complement depletion (10).
Ultraviolet light (UV) irradiation can lead to severe biologic damage, such as cutaneous inflammation, immunosuppression, and cancer. However, UV may also produce a biologic protective function, including the expression of many genes coding for transcription factors, growth factors, viral proteins, and proteases. The effect of UV irradiation on the immune system has been studied in the context of contact hypersensitivity, delayed-type hypersensitivity (11), and tumor rejection (12). UV irradiation can be used to treat a variety of skin diseases with which the therapeutic mechanism has been attributed to these immunosuppressive and/or anti-inflammatory properties.
On the basis of these observations, we hypothesized that irradiation with UV light could be one of the treatment modalities for cerebral vasospasm after subarachnoid hemorrhage, and we have previously reported the preventive effect of external UV irradiation on the development of chronic vasospasm in the rabbit common carotid artery (CCA) (13). Subsequently, we studied the preventive effect of external UV irradiation on the development of vasospasm and its mechanism by using a rat femoral artery model (14). This study revealed that a significant inhibition of chronic vasospasm by UV pretreatment occurred nearly parallel to a significant decrease in vascular smooth muscle cells. In these previous studies, the effect of UV on delayed vasospasm was examined under conditions in which UV irradiation was performed from the adventitial side of arteries before the arteries were exposed to blood. As the next step, anticipating possible clinical application, we developed an optical fiber technique in which the artery is irradiated with UV light in a cylindrically symmetric pattern via an endovascular approach. The purpose of this study was to investigate the preventive effect on vasospasm of this endovascular approach to administering UV light.
Methods
Animal Model
The experimental protocols used in this study were approved by the University Committee on Animal Resources of the University of Rochester. Sixty-three male New Zealand White rabbits weighing between 3.0 and 4.0 kg were used. Anesthesia was induced with an intramuscular injection of 25 mg/kg chlorpromazine hydrochloride and then an IV injection of 15 mg/kg sodium pentobarbital. Sodium pentobarbital solution was administered as a supplemental anesthesia as needed. Lidocaine hydrochloride (4 mg/kg) was infiltrated subcutaneously as a local anesthetic before skin incision. The right distal femoral artery was exposed and inserted with a 16-gauge IV catheter. A 3F microcatheter was introduced into the right CCA selectively through the IV catheter, and the tip of the microcatheter was placed at the level of the sixth cervical vertebra. A midline, longitudinal skin incision, measuring 4 cm in length, was made in the cervical portion of each animal. The right CCA was exposed and dissected free of loose connective tissue. Silastic sheaths measuring 3 cm in length were placed around the exposed CCAs of all rabbits. Fresh, non-heparinized blood (1 mL) was then drawn from the right femoral artery through a catheter placed for angiography. The blood was immediately placed into the perivascular space between the silastic sheath and CCA.
Angiography and Assessment of Vasospasm
On day 0, immediately before the operation on the CCA, an angiogram of the right CCA was obtained by using iohexol. To estimate arterial narrowing, we used the relative luminal diameter (RD), which was the ratio of the luminal diameter at the level of the silastic sheath to that at the fifth cervical vertebra. The luminal diameters of the CCAs were measured by a single observer (K.N.) in a blinded manner so that the observer was blinded to the type and duration of irradiation and the time interval after the treatment. The angiograms were converted into digitized images on a personal computer. Three different points along each artery were randomly selected on the image, and the diameter at each point was measured by using public domain imaging software (NIH image, version 1.62; National Institutes of Health, Bethesda, MD). The average of the three diameters was used to determine the final luminal diameter. We calculated a percentage of RD (%RD) relative to the baseline (day 0) for each rabbit.
The development of vasospasm was evaluated on day 2 because a maximum of vasospasm was observed 48 hours after blood exposure in our previous study (13) and in other reports (15, 16). The time course of change in the arterial diameter was consistent with the findings of previous reports. The mean arterial blood pressure, partial pressure of oxygen in arterial blood, and arterial carbon dioxide pressure were measured just after angiography on day 2 to examine the effect of these parameters on RD.
Preventive Effect of Endovascular UV Irradiation
Three experiments are described in the present study. The first experiment was to determine the optimal fluence of UV irradiation at 325 nm. The second experiment was to evaluate the effects of the UV irradiation on the artery sequentially on days 2, 9, and 30 from the standpoint not only of the preventive effect on vasospasm but also of the long-term effect on the arteries. In the third experiment, the preventive effect of visible light (442 nm) provided by the same laser system was examined as a control for the UV irradiation.
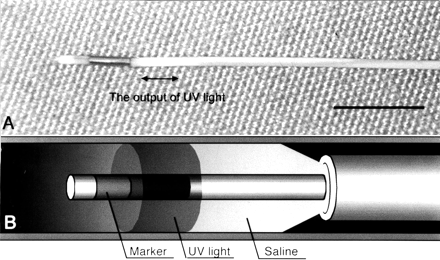
Thirty minutes after clot placement around the CCA, UV irradiation was performed by using a cylindrically diffusing optical fiber (Fig 1A), which was specially developed for this study (Lightstic 360; CardioFocus, Norton, MA). The fiber was introduced through a 3F catheter (SLIP-CATH infusion catheter; Cook, Bloomington, IN). The tip of the fiber was designed to deliver laser energy radially in a cylindrical pattern as shown in Figure 1A. The length of the diffusing section of the fiber was 2 mm, and the irradiation fluence rate at the vessel wall was estimated to be approximately 0.17 W/cm2. After a prescribed duration of irradiation at one location, both the catheter and the optical fiber were retracted by 2 mm. This procedure was repeated until the entire 3-cm length of the lesion was irradiated. Intraluminal blood was hand-flushed with saline during UV or visible light irradiation (Fig 1B). A helium-cadmium laser (Omnichrome 2074, 35/100 M; Melles Griot, Carsbad, CA) was used as the source of the UV and visible light.
Specially developed equipment used in this study.
A, Photograph of optic fiber. The fiber can deliver laser energy and diffuse UV or visible light in a cylindrical pattern from a diffusing section. The diameter of the optical fiber is 0.49 mm (0.019 in). Scale bar, 5 mm.
B, Illustration of endovascular UV irradiation. The blood was hand-flushed with saline to irradiate the entire arterial wall homogeneously with UV or visible light.
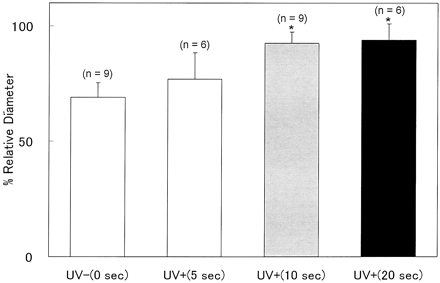
To determine the optimal UV irradiation time, 30 animals were randomly assigned to one of four groups corresponding to 0 seconds (n = 9), 5 seconds (n = 6), 10 seconds (n = 9), and 20 seconds (n = 6) of irradiation. For all animals, the blood clot was placed around the CCAs and angiograms were obtained on days 0 and 2. From these results, the irradiation time that maximized the preventive effect and minimized the potential damage was determined to be optimal.
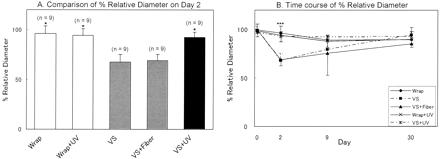
To examine the time course of response among different treatment groups, rabbits were randomly divided into five groups before wrapping the CCA in a silastic cuff: 1) silastic cuff only (wrap group, n = 9); 2) clot placement (vasospasm [VS] group, n = 9); 3) silastic cuff only and UV treatment (wrap + UV group, n = 9); 4) clot placement and fiber manipulation only (VS + fiber group, n = 9); and 5) clot placement and UV treatment (VS + UV group, n = 9). The wrap group (n = 9) and the VS + UV group (n = 9) were identical to the groups corresponding to no irradiation (n = 9) and optimal time of irradiation (n = 9) in the previous experiment, respectively. Therefore, we used an additional 27 rabbits in this experiment. Each subgroup was subjected to angiography on days 0 (n = 45) and 2 (n = 45) and at one of three end points: day 2 (n = 16), day 9 (n = 14), and day 30 (n = 15).
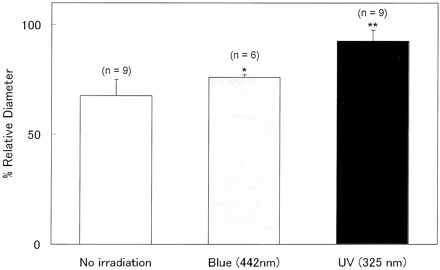
Six animals underwent visible light (442 nm) irradiation 30 minutes after clot placement as a control for comparison with UV irradiation. The intensity of the visible light was adjusted to the same level as that of the UV. For all animals in which the blood clot was placed around the CCAs, angiograms were obtained on days 0 and 2.
Histopathologic Examination
The rabbits were killed by overdose of sodium pentobarbital after the final angiography. The right CCAs were removed, embedded in paraffin, stained with hematoxylin and eosin, and observed under a light microscope.
Statistical Analysis
The data are presented as the mean ± SD. For statistical comparisons, analysis of variance was performed and then a Scheffé’s test was conducted for multiple comparisons if a significant probability was reached. Correlations between physiological parameters and RD were statistically analyzed by using Pearson’s correlation coefficient. A level of P < .05 was considered significant. Correlations were considered to exist when the absolute value of the sample correlation coefficient exceeded 0.4. The software Statview for Macintosh (SAS Institute Inc., Cary, NC) was used for these purposes.
Results
Vasospasm in the Rabbit CCA Model
Physiological parameters measured just after angiography on day 2 in each group are listed in the Table. There were no significant differences among the groups in terms of mean arterial blood pressure, partial pressure of oxygen in arterial blood, and arterial carbon dioxide pressure. The data showed no obvious correlations between these physiological parameters and the RD under spontaneous respiration. This result confirmed that our vasospasm model could be used under spontaneous respiration.
Summary of physiological parameters
Determination of the Optimal Irradiation Time
Figure 2 shows that UV treatment for irradiation times of 10 or 20 seconds significantly inhibited the development of vasospasm compared with no irradiation (P < .001). The %RD from angiograms revealed the preventive effect to plateau at an irradiation duration of 10 s. No significant difference was observed between groups in which the irradiation time was 10 or 20 seconds (P = .869). To reduce the possibility of any unanticipated adverse effects of UV irradiation at this wavelength and to minimize the treatment time, we selected 10 rather than 20 seconds as the optimal irradiation time.
Bar graph shows the preventive effect of UV on the development of vasospasm for different irradiation time periods. Four groups are shown, for which the irradiation time was 0, 5, 10, or 20 seconds. UV irradiation with durations of 10 and 20 seconds significantly prevented the development of vasospasm (P < .001). Values represent the ratio of the relative diameter in each group on day 2 to that on day 0. The preventive effect seems to be close to maximum at an irradiation period of 10 seconds. Bars represent mean ± SD. *, P < .05 compared with no irradiation.
Preventive Effect of UV Light versus Control Groups
The VS and VS + fiber groups exhibited statistically significant reductions in %RD by 27% when compared with the wrap and the wrap + UV groups (Fig 3A). The values for the VS and VS + fiber groups did not differ significantly. This result shows that manipulation of a catheter and an optical fiber did not influence the degree of vasospasm. The magnitude of vasospasm was significantly attenuated in animals subjected to UV treatment. UV irradiation (VS + UV group) reduced the degree of vasospasm by 26% when compared with the VS + fiber group. On days 9 and 30, there were no significant differences among all groups, although %RD in the VS and VS + fiber groups was smaller than in the other groups on day 9 (Fig 3B). No adverse effects such as perforation, intramural hematoma, or dissection occurred during this period.
Bar graph shows values that represent the ratio of the RD in each group on each point to that on day 0 and represent mean ± SD.
A, Preventive effect of UV on the development of vasospasm among different treatment groups on day 2. UV treatment significantly inhibited vasospasm (P < .001 compared with the VS + fiber group). Bar graph shows that the preventive effect on vasospasm was not due to manipulation (VS + fiber group) but to UV irradiation (VS + UV group). *, P < .05 compared with the VS + fiber group.
B, Time course of %RD among different treatment groups.
Preventive Effect of Visible Light
Visible light also significantly prevented the development of vasospasm by 8% compared with the VS + fiber group (P = .045) (Fig 4). However, the effect was significantly less than that of UV light. There was a significant difference in the magnitude of the preventive effects induced by UV versus visible light (P < .001).
Bar graph shows the preventive effect of UV or visible light on the development of vasospasm. Values represent the ratio of the RD in each group on each point to that on day 0 and represent mean ± SD. Although visible light significantly prevented vasospasm (P = .045), UV light was more effective. *, P < .05; **, P < .01 compared with the no irradiation (VS + fiber) group.
Histologic Analysis
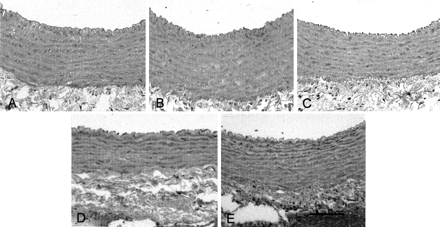
On day 2, the CCAs subjected to autologous blood (VS and VS + fiber groups) exhibited subendothelial thickening and the corrugation of the internal elastic lamina (Fig 5A and B, respectively) compared with those wrapped by cuffs only (wrap group, Fig 5C). In contrast, the internal elastic lamina of the UV-treated vessels (wrap + UV and VS + UV groups) had a smooth appearance, and morphologic changes in the vascular structure did not occur (Fig 5D and E, respectively). The corrugation of the internal elastic lamina in the VS and VS + fiber groups was almost resolved on day 9 (data not shown). No disruption, intramural hematoma, dissection, or necrosis was seen in the vascular walls, regardless of UV irradiation at day 30 (data not shown).
Photomicrographs show cross sections of rabbit CCAs on day 2. Hematoxylin and eosin stain was used. Placement of autologous blood around CCAs induced folding of the endothelial surface, the corrugation of the internal elastic lamina, contraction of smooth muscle cells, and thickening of the arterial wall compared with no blood placement. In the UV-treated vessel, there is less folding of the internal elastic lamina and the smooth muscle cells appear relaxed. Scale bar, 100 μm.
A, VS group.
B, VS + fiber group.
C, Wrap group.
D, Wrap + UV group.
E, VS + UV group.
Discussion
We previously reported the preventive effect of UV on vasospasm in the rabbit CCA model (13). In the previous study, UV irradiation was administered from the outside of the artery immediately before placement of the clot. Thus, the potential clinical relevance of the findings was limited in that the UV light was applied before blood immersion. Further, because UV light does not penetrate the blood clot, it was not possible to irradiate the vessel adventitiously after blood immersion. Irradiation of a vessel adventitiously after blood immersion would require removing the blood clot, which would limit the validity of the animal model itself by altering the time course of development of the vasospasm. Therefore, in the present study, arteries were irradiated with UV light via an endovascular approach after blood immersion, and, in this more clinically relevant model, the UV irradiation was effective in the prevention of vasospasm.
Our previous investigation, in which we used a rat femoral artery model (14), indicated that the number of vascular smooth muscle cells in the media was reduced under conditions in which the preventive effect of UV pretreatment for vasospasm was apparent. Pharmacologic evidence showed that the contractile responses of UV-irradiated vessels to high potassium or phenylephrine were markedly attenuated in comparison with control arteries. These results led us to hypothesize that UV irradiation may prevent development of chronic vasospasm by attenuating contractility secondary to UV-induced cell death, which seemed to be different from a typical apoptotic cell death and to agree with the characteristics of caspase-independent cell death, which has been documented by recent reports (17, 18). This type of cell death shows the cytoplasmic features of apoptosis (cell shrinkage, decrease in mitochondrial transmembrane potential, and phosphatidylserine externalization) without the nuclear features (chromatin condensation, appearance of single-stranded DNA, DNA fragmentation, and cleavage of poly[adenosine diphosphate ribose] polymerase) (19). However, it is difficult to determine the type of cell death induced by UV irradiation in the present study because it is evident that there are multiple pathways (20), and further investigation is required.
Absorption of UV radiation by endogenous chromophores elicits a number of biologic responses. UV light is divided into three components on the basis of wavelength: UVA (wavelength, 320–400 nm); UVB (wavelength, 290–320 nm); and UVC (wavelength, 200–290 nm). The UVB component causes cellular DNA damage, predominantly through direct absorption by DNA, whereas the UVA component is absorbed by endogenous chromophores other than DNA. UVA-induced oxidative damage has been reported to occur in lipids (21), coenzymes (22), and DNA (23). Consequently, cellular responses are evoked for the purposes of antioxidant defense (24), detoxification, and repair, but sufficient damage leads to cell death (25). Reactive oxygen species that activate the effector caspases through release of cytochrome c from mitochondria (26, 27) also play an important role in induction of necrosis and caspase-independent cell death (20, 28). Although reactive oxygen species may be initiated by clot lysis after subarachnoid hemorrhage and may contribute to vasospasm, our data suggest that UV-induced cell damage via reactive oxygen species is the dominant effect, because cell death was observed in the UV-treated vessels. Some reports in dermatology (29, 30) revealed that photobiologic effects were more evident in response to UV versus visible light beyond 400 nm. A recent study in a murine tumor cell spheroid model showed that rates of photochemical oxygen consumption were significantly greater during 325-nm irradiation than they were in response to 442-nm irradiation at the same fluence rates (31). These findings are consistent with our result that the preventive effect in vasospasm was less effective with 442 nm than with 325 nm of light.
Intracranial injection of autologous blood would have an excellent chance of generating vasospasm similar to that seen after rupture of an intracranial aneurysm. Research on cerebral vasospasm has involved the use of cerebral arteries in animals including the rat, cat, rabbit, dog, pig, and monkey (32). However, because of their small size and intracranial location, it is often difficult to perform angioplasty or injection of antispasmodic agents in these models (15, 16). This has led several investigators to use extracranial arteries. Okada et al (33) first introduced a rat femoral artery model of vasospasm, and this has been followed with the use of the cervical carotid artery in rabbits (13, 15, 16, 34, 35) and dogs (36, 37).
Intracranial and extracranial arteries differ fundamentally in their endothelial permeabilities, the nature of the adventitial matrices, and their responses to certain vasoactive agents. Despite these differences, vasospasm in extracranial arteries follows the same time course and has angiographic, morphologic, and pharmacologic features similar to those of intracranial vasospasm (32, 33, 36). The possible effect of mechanical dilation by manipulations similar to percutaneous transluminal angioplasty should be considered under conditions in which the diameter of a vessel is smaller than that of the catheter that delivers the optical fiber and enables us to flush blood appropriately with saline. However, our control experiments with catheter and fiber manipulation in the absence of irradiation indicated that this was not a significant factor in our experiments.
In the present study, endovascular UV irradiation with a fluence rate of 0.17 W/cm2 for 10 seconds prevented development of vasospasm in the CCA after blood exposure. The dose-response relation with respect to UV irradiation time showed that the preventive effect seemed to reach a plateau after 10 seconds of irradiation. The total fluence of 1.7 J/cm2 corresponded to 1.7 times that used in our previous study, in which UV light was delivered to the artery from the outside (13). Even if the vessel lumen was replaced with saline temporarily through flushing, flowing blood would reduce UV energy delivered to the vessel wall under conditions of intravascular irradiation. Taking this into consideration, it is possible to conclude that these two results are not inconsistent with respect to the energy required to achieve the prophylactic effect in this model. An increased laser energy density might not only have little additional preventive effect on vasospasm but may also enhance cytotoxicity. However, we note that there were no histologic differences between the groups treated for 20 versus 10 seconds, and no severe complications such as perforation, intramural hematoma, or dissection were observed in any of the treated vessels. The advantages of UV irradiation via the endovascular approach are that focal therapy is possible, target vessels can be more selectively treated, and there is virtually no risk of perforation, which is occasionally encountered in association with percutaneous transluminal angioplasty. Intraarterial UV irradiation applied before or after surgical clipping of a ruptured aneurysm might be useful to prevent vasospasm. Furthermore, endovascular UV treatment during or after coil embolization for a ruptured aneurysm may also be useful to prevent vasospasm. Because of the differences between cerebral and extracranial arteries, further investigations using intracranial models are required to determine in detail the therapeutic window and the optimal conditions of this treatment. Long-term effects of UV light on the irradiated arteries with regard to function and cytotoxicity should also be clarified before clinical application.
Conclusion
UV irradiation via an endovascular approach successfully prevented the development of experimental vasospasm in a rabbit CCA model. Our results suggest that this treatment may thus be useful as an additional therapy for delayed cerebral vasospasm accompanying subarachnoid hemorrhage.
Acknowledgments
The authors are especially grateful to Dr. Arvin Robinson, Chair of the Department of Radiology at the University of Rochester, for significant support of the project in the form of resources, laboratory space, and encouragement during the conduct of these studies. The authors also thank the members of Department of Laboratory Animal Medicine at the University of Rochester Medical Center, Dr. Toshio Moritani, Makiko Nakai, and Yoshiko Kon, for assistance in various aspects of the experiments. The dedicated help of William J. Quinlan and David L. Conover is deeply appreciated. Dr. Ed Sinofsky of CardioFocus, Inc., provided critical guidance in the design of the optical fibers.
Footnotes
Presented in part at the 39th Annual Meeting of the American Society of Neuroradiology, Boston, MA, April 21–27, 2001.
Supported in part by a grant from Toshiba America Medical Systems (to Y.N.).
References
- Received February 25, 2002.
- Accepted after revision July 15, 2002.
- Copyright © American Society of Neuroradiology