Abstract
BACKGROUND AND PURPOSE: Traumatic neuroma, an attempt by an injured nerve to regenerate, may present as a palpable nodule or an area sensitive to touch (trigger point) after neck dissection. The purpose of this study was to identify CT characteristics of traumatic neuroma in four patients after neck dissection.
METHODS: Between April 1995 and November 1998, the CT studies in three men and one woman (ages, 45–64 years) who had had a radical neck dissection and a nodule posterior to the carotid artery were reviewed retrospectively. CT was performed 1.5 to 6 years after neck dissection with clinical correlation and/or pathologic examination. Three patients had squamous cell carcinoma of the upper aerodigestive tract and one had a primary parotid adenocarcinoma.
RESULTS: Three patients with a traumatic neuroma had a centrally radiolucent nodule with peripherally dense rim and intact layer of overlying fat, which was stable on CT studies for 1 to 2 years. One of these had a clinical trigger point. The fourth patient with a pathologically proved traumatic neuroma mixed with tumor had intact overlying fat, but the nodule lacked a radiolucent center and was not close to the carotid artery.
CONCLUSION: The CT findings of a stable nodule that is posterior but close to the carotid artery with central radiolucency, a dense rim, and intact overlying fat, combined with the clinical features of a trigger point and a lack of interval growth, strongly suggest the diagnosis of traumatic neuroma.
A traumatic neuroma is a thwarted attempt by a nerve, injured or severed by prior trauma or surgery, to regenerate, resulting in a tangle of neural fibers and connective tissue (1). Traumatic neuromas usually present as palpable nodules, painful to the touch, which may be associated with paresthesia. Early in the 19th century, interest in traumatic neuromas was stimulated by findings in war-wounded veterans with amputated limbs in whom painful stumps and traumatic neuromas developed (2). With the emergence of radical neck dissection, information has been available as to the gross and histologic structure of cervical traumatic neuromas.
Most of the prior studies reported in the surgical literature are case reports describing the presentation and location of traumatic neuromas after neck dissection (3–8). They reveal that the nodules occur posterior to the internal and common carotid arteries (ICA and CCA) (4) and most likely result from injury to the peripheral sensory nerves. Traumatic neuromas may arise from the great auricular nerve (C2, C4), the cutaneous cervical nerves (C2, C3), the supraclavicular nerve (C3, C4), and the superficial branches of the cervical plexus transected during radical neck dissection (5). After a parotidectomy, these nodules typically occur near the scar in the region of the posterior border of the sternocleidomastoid muscle (6). We are unaware of prior radiologic descriptions of traumatic neuromas in the head and neck.
The surgical literature states that traumatic neuromas seldom require excision unless they are symptomatic (6). Close et al (9) state that excision is unnecessary for patients with prior neck dissection and traumatic neuromas in typical locations posterior to the carotid artery near the second cervical nerve. Fine-needle aspiration may be nondiagnostic (7) or made difficult by acute tenderness (6). An accurate diagnosis of traumatic neuromas may obviate surgical resection.
In the past, traumatic neuromas were either clinically undiagnosed or misdiagnosed, causing patients with prior neck dissections unnecessary anxiety and the need to undergo surgical excision (2). The purpose of this study was to identify the CT characteristics of traumatic neuromas that could help distinguish them from recurrent tumor in patients with prior neck dissection.
Methods
A review of the computerized medical records at the University of Pittsburgh Medical Center from April 1995 to November 1998 revealed four patients (three men and one woman, 45–64 years old) who had undergone radical neck dissection in whom contrast-enhanced CT studies of the neck revealed a nodule in the expected location of a traumatic neuroma, and in whom clinical correlation, pathologic proof, or comparison CT studies were available. The initial CT scans were obtained 1.5 to 6 years after neck dissection. Three patients had squamous cell carcinoma of the upper aerodigestive tract and one had a primary parotid adenocarcinoma. All four patients underwent a modified type 1 radical neck dissection with preservation of the spinal accessory nerve. Neck dissections were performed in 445 patients at our institution during this time period. Of these patients, recurrent tumor developed in four and was reexcised. One traumatic neuroma was found incidentally during surgery and noted on the pathology report (no CT scan available).
Results
The contrast-enhanced CT scans of three patients revealed a centrally radiolucent nodule with a dense rim and an intact layer of overlying subcutaneous fat. Two of the three nodules were posterolateral and close to the CCA (Figs 1 and 2) and one was posterolateral and close to the ICA (Fig 3). These findings were stable over 1 to 2 years. One of these patients had a primary parotid adenocarcinoma and a clinical trigger point, typical of traumatic neuroma (Fig 3). The fourth patient had intact overlying fat, but the nodule lacked a radiolucent center (Fig 4A). This nodule was posterolateral to but separate from the ICA and was pathologically proved to be a traumatic neuroma mixed with tumor (Fig 4B and C).
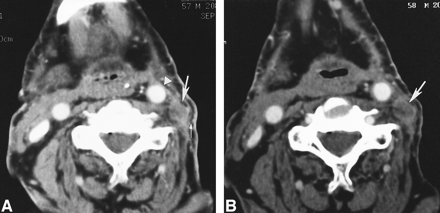
59-year-old man with squamous cell carcinoma of the larynx after left-sided modified radical neck dissection (type 1) with sparing of the spinal accessory nerve.
A, Axial contrast-enhanced CT scan of the neck shows a nodule (large arrow) with a radiolucent center, peripherally dense rim, and an intact layer of overlying fat (small arrow). The nodule lies posterolateral and close to the left CCA (arrowhead). The left sternocleidomastoid muscle and left internal jugular vein are absent.
B, Axial contrast-enhanced CT scan of the neck obtained 1 year after A shows the nodule (arrow) to be minimally increased in size, which is highly atypical for tumor.
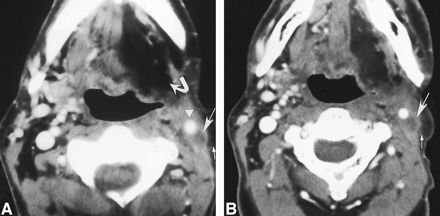
45-year-old man after left-sided modified radical neck dissection (type 1) with sparing of the spinal accessory nerve 1 year after a left partial glossectomy and selective neck dissection (types 1 and 2) for squamous cell carcinoma of the tongue.
A, Axial contrast-enhanced CT scan shows a nodule (large straight arrow) with a radiolucent center, peripherally dense rim, and an intact layer of overlying subcutaneous fat (small arrow). This nodule is posterolateral and close to the left CCA (arrowhead). Left-sided hypoglossal denervation (curved arrow) is also present.
B, Axial contrast-enhanced CT scan 1 year after A shows this nodule (large arrow) and its intact layer of overlying fat (small arrow) remain unchanged.
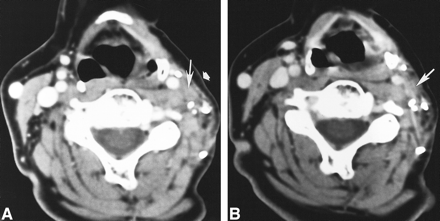
54-year-old woman 4 years after left-sided modified radical neck dissection with sparing of the spinal accessory nerve for parotid adenocarcinoma now with a trigger point.
A, Axial contrast-enhanced CT scan shows a nodule (long arrow) with faint central radiolucency, a peripherally dense rim, and an intact layer of overlying fat (short arrow). This nodule lies posterolateral and close to the left ICA.
B, Axial contrast-enhanced CT obtained 2 years after A shows this nodule (arrow) to be stable.
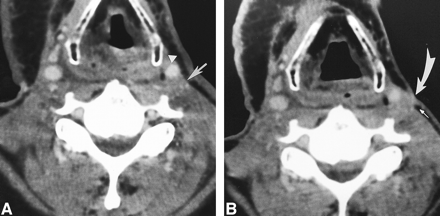
64-year-old man with a right-sided neck mass 7 months after right-sided modified radical neck dissection and knife excision of zones I through V for squamous cell carcinoma of the larynx.
A, Axial contrast-enhanced CT scan reveals a nodule (arrow) posterolateral to but separate from the right ICA (arrowhead). The nodule lacks a radiolucent center and peripherally dense rim.
B, Microscopic section of the nodule reveals tangled and interwoven neurofibrils (arrowheads) mixed with connective tissue, typical of a traumatic neuroma (hematoxylin-eosin, ×400).
C, Another microscopic section of the nodule also reveals other areas containing abnormally mitotic nuclei (arrows), typical of carcinoma mixed with areas typical of traumatic neuroma (hematoxylin-eosin, ×400).
Discussion
Traumatic neuromas represent an exaggerated response to nerve injury, resulting in reactive hyperplasia; they are not neoplastic in origin (1). Probably the most accurate description of traumatic neuroma has been offered by Huber and Lewis (10). A neuroma (traumatic) indicates an attempt, which is thwarted or blocked by scar tissue, on the part of the neuraxes of a divided nerve to seek the distal segment and thus complete nerve repair. When blocked, the regenerating neuraxes form spirals and end disks and become irregularly dispersed throughout the connective tissue of the bulb. The regenerating neuraxes react on the connective elements of the bulb, which as a consequence, increase in number and maintain their embryonal characteristics longer than is normally the case.
Traumatic neuromas appear grossly as firm, oval, whitish nodules that are rarely larger than 2 cm (1). At sectioning, they have a dense fibrous appearance with little vascularity. A nerve may terminate at the upper pole of the mass (1). Although not encapsulated, the outer layer of fibrous tissue is often inseparable from the surrounding scar, and microscopically, an outer layer of connective tissue is continuous with the perineurium of the intact nerve trunk (11).
Neck dissection, or cervical lymphadenectomy, is a procedure for eradicating metastases to the regional lymph nodes of the neck (12). A radical neck dissection includes removal of all ipsilateral cervical lymph nodes from the level of the body of the mandible to the clavicle (12), including the spinal accessory nerve, internal jugular vein, and sternocleidomastoid muscle. This procedure is indicated for extensive lymph node metastases or extension of tumor beyond the capsule of the node(s) to involve the spinal accessory nerve and internal jugular vein. The motor branches of the cervical plexus and brachial plexus lie just beneath the fascia overlying the splenius muscle. During dissection of the posterior triangle, these nerves may be transected (12).
Traumatic neuromas appear as tender nodules in the lines of incision 1 to 10 years after neck dissection (5). Patients with traumatic neuromas may present with paraesthesia distributed over the injured area. Painful hypersensitivity to light tactile stimuli (dysesthesia), or a trigger point, may be a prominent feature. Tenderness on percussion, on pressure, and on distortion of surrounding tissues is commonly present (11). When a palpable nodule occurs in a patient who had prior carcinoma resection, tumor recurrence is of primary concern. Clinical correlation of a palpable nodule sensitive to touch, associated with a trigger point or with paresthesia, will aid in the diagnosis of traumatic neuroma.
Traumatic neuromas are known to occur elsewhere in the body. Intraoral neuromas tend to form in the lower jaw after fracture or surgery whereas neuromas of the sympathetic nervous system may occur after amputation of the cystic duct during cholecystectomy (11). Traumatic neuromas typically occur after limb amputation, and may be divided into two categories: 1) spindle neuromas, which are caused by chronic friction or irritation to a nondisrupted intact nerve trunk and that appear as focal fusiform internal areas of fibroinflammatory reaction, and 2) terminal neuromas, which arise after transection of a nerve (13). CT characteristics of neuromas that occur after limb amputation include focal irregularities or swelling of the distal resected nerve end. The pattern of contrast enhancement has not proved useful (14).
The characteristics of traumatic neuromas in the neck in our series included a nodule with a radiolucent center, peripherally dense rim, and an intact layer of overlying fat that remained stable over time. A traumatic neuroma may grow very slowly, unlike a tumor. A traumatic neuroma would not be expected to shrink in response to radiation therapy or chemotherapy. The CT findings in conjunction with the clinical setting may distinguish traumatic neuroma from recurrent tumor.
In the setting of a palpable nodule posterior to the carotid without a typical trigger point, treatment may include observation with a repeat contrast-enhanced CT study in 3 months to assess stability if the nodule shows the characteristics described earlier. Another option may include 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), which has been shown to be helpful in the elucidation of unknown lesions in 25% to 65% of patients with metastases and no identifiable primary lesion (15). In patients who have not undergone surgery, the sensitivity for detecting head and neck carcinoma nodal disease ranges from 67% to 91%, with a specificity of 88% to 100%. However, FDG is limited when tumor deposits are less than 4 mm (false-negative result) and by uptake of tracer in reactive lymph nodes (false-positive result). Another cause of false-negative PET results is complete nodal necrosis, because there is not enough viable tumor for FDG uptake (15). Another issue is the timing of FDG-PET after radiation therapy; FDG-PET is not accurate for at least 1 month after therapy. False-positive findings may also occur with blood pool activity in the jugular vein, which may be distinguished on the long axis. Additionally, PET is not readily available at many institutions. Recent in vitro investigations suggest the use of proton MR spectroscopy markers choline and creatine in the detection of malignant disease in the head and neck, although microscopic foci of tumor cannot be excluded (15). Still another approach may include fine-needle aspiration; however, for similar reasons, sampling error may lead to a false-negative result. Management would depend on the clinical situation, such as tumor staging, surgical findings, and interval since surgery.
A thrombosed internal jugular vein, necrotic lymph node, or tumor deposit may mimic the appearance of a traumatic neuroma on CT studies. However, in contrast to a thrombosed internal jugular vein, a traumatic neuroma would not appear as a persistent tubular structure on multiple images. The location of traumatic neuromas, posterolateral and close to either the CCA or ICA, would be unusual for lymphadenopathy in a patient with prior neck dissection. On CT scans, a soft-tissue tumor deposit may resemble a traumatic neuroma, and tumor in a traumatic neuroma (Fig 4A) may initially resemble an uncomplicated traumatic neuroma. We encountered a patient who had had a radical neck dissection in whom a heterogeneous nodule posterolateral to the ICA developed (Fig 5A), which enlarged over the course of a year, invading the overlying subcutaneous fat (Fig 5B). The lack of a radiolucent center, fat invasion, and growth pattern all made traumatic neuroma unlikely. The lesion was pathologically proved to be recurrent tumor. In both cases, the nodule lacked a radiolucent center. Furthermore, recurrent tumor or cervical lymphadenopathy would not typically present with symptoms of a trigger point or paresthesia, and the tumor would enlarge over time. Thus, sequential contrast-enhanced CT of the neck in combination with clinical correlation may help to accurately diagnose traumatic neuromas and avert unnecessary surgical resection.
52-year-old man with a possible neck mass after prior bilateral radical neck dissections for squamous cell carcinoma of the tongue.
A, Axial contrast-enhanced CT scan shows a heterogeneous nodule (arrow), posterolateral to the left ICA (arrowhead), which lacks a radiolucent center and peripherally dense rim.
B, Axial contrast-enhanced CT scan 3 months after A shows the nodule (curved arrow) to be enlarged and invading the overlying subcutaneous fat (straight arrow). This nodule was pathologically proved to be recurrent squamous cell carcinoma.
Our retrospective review focused only on CT studies. However, we hypothesize that, on MR studies, traumatic neuromas in the neck would appear posterolateral to the CCA or ICA and show peripheral rim enhancement. Most important, follow-up MR imaging would reveal a stable nodule.
In our series of patients with neck dissections, one may conjecture a correlation with the type of neck dissection and the likelihood of traumatic neuroma formation as opposed to tumor recurrence. Three patients with traumatic neuromas had undergone modified neck dissection with sparing of the spinal accessory nerve (type 1). The traumatic neuromas were present on CT scans obtained 1.5 to 4 years after these modified dissections. The patient with a mixed traumatic neuroma and tumor also had undergone a modified (type 1) neck dissection, and the mixed nodule appeared 7 months after surgery. The patient with recurrent tumor had undergone a radical neck dissection, and this nodule was present on CT scans 3 months after surgery and enlarged over a period of 6 months. In our series of four patients, one might assume a relationship between traumatic neuroma formation and modified neck dissection. However, there may be a stronger correlation between staging, the type of neck dissection, and tumor recurrence. Patients with radical neck dissections at our institution tend to have more infiltrative, adherent tumor and may be at greater risk for tumor recurrence. Tumor recurrence may occur earlier than traumatic neuroma formation, and thus be detected earlier on CT studies. The association between the type of neck dissection and traumatic neuroma formation may be a subject for further study in a larger group of patients.
Several types of treatment, both surgical and nonsurgical, have been advocated for amputation neuromas (8). These neuromas respond well to simple excision and embedding of the proximal stump away from scar tissue, which may retract and cause pain (4, 5). Nonsurgical methods of treatment are less successful and include local infiltration with steroids, sympathetic block, and percussion with mechanical vibrators (11). A recent report in the surgical literature states that traumatic neuromas seldom require excision unless symptomatic; for example, if pressure from clothing causes discomfort (6), Close et al (9) state that excision may be therapeutic and useful for diagnosis but is not necessary for such lesions in their typical location (posterior to the carotid artery near the second cervical nerve) and associated with paresthesia or unpleasant tingling. A combination of radiologic and clinical diagnosis of traumatic neuroma may obviate surgical resection of a palpable nodule.
Conclusion
Typical CT characteristics of traumatic neuromas in a patient with a prior neck dissection include a nodule with a radiolucent center, peripherally dense rim, and an intact layer of overlying fat that is stable over time. These nodules occur posterolateral but close to either the CCA or ICA. In combination with these CT findings, clinical correlation showing a palpable mass associated with a trigger point or paresthesia will help prevent misidentification of a traumatic neuroma as a tumor and obviate unnecessary surgery.
Footnotes
1 Presented at the annual meeting of the American Society of Neuroradiology, San Diego, May 1999.
↵2 Address reprint requests to Jane L. Weissman, MD, Department of Radiology and Otolaryngology, Oregon Health Sciences University, 3181 SW Sam Jackson Park Rd, Mail Code CR-135, Portland, OR 97201.
- Received August 23, 1999.
- Accepted after revision March 14, 2000.
- Copyright © American Society of Neuroradiology