Abstract
BACKGROUND AND PURPOSE: The possibility of treating intracranial vasospasm has increased the significance of its diagnosis and follow-up; however, so far, no ideal method is available. The goal of this study was to assess the accuracy of MR angiography versus intraarterial angiography (IA-DSA) in detecting vasospasm.
METHODS: The study included 42 patients with acute spontaneous subarachnoid hemorrhage (SAH). Serial MR angiograms (minimum, two per patient within 10 days after the event; total, 149) were obtained prospectively using a 3D time-of-flight technique covering the circle of Willis at 0.5 T. Forty-seven MR angiograms could be compared with intraarterial angiograms obtained within 24 hours of MR angiography. Vascular narrowing on both studies was rated consensually by two pairs of neuroradiologists using a scale from 0 (no narrowing) to 3 (severe narrowing). Categories 0 and 1 were considered an absence of vasospasm and categories 2 and 3 a presence of vasospasm.
RESULTS: Agreement between MR angiography and IA-DSA (assessed with weighted κ statistics) was substantial for the middle and anterior cerebral arteries (MCA and ACA) but moderate for the internal carotid artery (ICA). The sensitivity, specificity, accuracy, and positive and negative predictive values of MR angiography for detecting patients with vasospasm were 92%, 98%, 96%, 92%, and 98%, respectively. Considering each vessel separately, specificity was high for all locations (95–99%) and sensitivity was excellent for the ACA (100%) but poorer for the ICA (25%) and MCA (56%).
CONCLUSION: MR angiography at 0.5 T is capable of identifying vasospasm after acute SAH but is less sensitive than IA-DSA for depicting vasospasm in the ICA and MCA.
The rate of successful occlusion of ruptured cerebral aneurysms has increased dramatically with the use of microsurgical and endovascular techniques. However, pre- and/or postoperative vasospasm remains a major complication, producing ischemic deficits and accounting for a significant morbidity after subarachnoid hemorrhage (SAH) (1, 2). Prophylactic use of nimodipine has been shown to prevent ischemic consequences of vasospasm and to improve clinical outcome (3). When vasospasm develops despite treatment with calcium channel blockers, the now-classical triple H therapy (hemodilution, hypertension, and hypervolemia) is generally used (4, 5). Other therapeutic strategies—such as local intraarterial infusion of papaverine, balloon angioplasty, and, more recently, intraaortic counterpulsation—are also available (6, 7). The diagnosis of vasospasm is, therefore, particularly important and should ideally be made before the development of potentially irreversible symptoms.
For many years, intraarterial angiography (IA-DSA) was the only way to positively establish the diagnosis of vasospasm in a patient with worsening neurologic function after SAH (1, 2, 8). However, angiography is an invasive procedure, and, in patients with SAH and vasospasm, carries a risk as high as 1.8% for transient or permanent neurologic complications (9). Today, transcranial Doppler sonography is largely accepted as a convenient method for evaluating noninvasively the status of intracranial arteries in patients after SAH (9, 10). Recordings of blood flow velocity enable the identification of vasospasm, but the accuracy of transcranial Doppler sonography is limited by several drawbacks, such as an inability to find an adequate acoustic window or to address peripheral arteries and collaterals, and by the presence of such confounding factors as arterial blood pressure, hematocrit, intracranial pressure, and patients' age, all of which alter the relationship between increased blood velocity and angiographic vasospasm (11–13). An alternative is to identify significant vasospasm by measuring local cerebral blood flow (CBF). Positron emission tomography and, more commonly, single-photon emission computed tomography or xenon-enhanced CT are suitable for this goal, but the assessment of vasospasm remains incomplete, as these techniques do not show the morphology of the vessels (14, 15).
In the search for an ideal technique, MR imaging may be a good candidate, as it can address both the angiographic side for direct identification of arterial vasospasm and the functional side by measuring the regional CBF and volume (16–20).
The aim of this study was to compare the performance of MR angiography with IA-DSA in diagnosing vasospasm in patients with acute spontaneous SAH.
Methods
Study Design
We designed a prospective study to evaluate the accuracy of MR angiography in diagnosing angiographic vasospasm and to test the feasibility of using MR angiography as a follow-up tool for detecting vasospasm. Systematic MR angiography was performed in each patient with acute SAH the day after admission and subsequently every 3 to 4 days until aneurysmal occlusion, which was achieved during the subacute phase (typically at the end of the second week after SAH). However, because patient or relative consent was needed for each MR angiographic study, it was not possible to strictly follow this timing in all subjects. If vasospasm was clinically questionable in case of neurologic worsening, an additional MR angiogram was performed. IA-DSA studies were the standards of reference to which MR angiograms were compared. Unless the patient underwent IA-DSA before admission, preoperative IA-DSA was generally performed during the second week after SAH, with some additional examinations in the case of highly symptomatic vasospasm performed before making a treatment decision. In a few cases, MR angiography and IA-DSA were performed at a time remote from the acute event: 1) to determine the cause of SAH when it could not be established in the acute phase on the first IA-DSA study; 2) to evaluate the success of aneurysmal occlusion; 3) to assess a second, unruptured aneurysm; or 4) to determine whether vessel narrowing was due to hypoplasia or a preexisting condition.
Study Population
The study group included 42 patients with acute spontaneous SAH confirmed by lumbar puncture and/or CT. There were 20 men and 22 women, aged 23 to 77 years (mean age, 46 ± 13 years), who were enrolled consecutively only if they had undergone a minimum of two MR angiographic examinations within 10 days after SAH (encompassing the vasospasm period) and who had at least one IA-DSA study available for comparison with the MR angiogram. No patient was excluded because of technically inadequate examinations. The origin of SAH could not be identified in eight cases; in all other patients, an aneurysm was found and was the presumed cause of the SAH. Aneurysms were located in the anterior communicating artery (AComA) (n = 15), the internal carotid artery (ICA) (n = 9), the middle cerebral artery (MCA) (n = 8), and the posterior circulation (n = 2). All patients received prophylactic therapy with intravenous nimodipine for 14 days, followed by oral administration in the absence of symptomatic vasospasm. Ruptured aneurysms were treated during the subacute phase either by a surgical or endovascular approach. As a consequence, 94% of MR examinations were performed in the preoperative period.
MR Angiography
A total of 149 MR angiograms (mean, 3.6 ± 3 per patient) were obtained on a 0.5-T superconductive system with a quadrature receive-only head coil. A standardized 3D time-of flight (3D-TOF) technique was used with a 30-mm-thick transverse slab divided into 30 partitions and centered on the circle of Willis. The sequence parameters were as follows: 32/8/2 (TR/TE/excitations), 17° flip angle, 256 × 192 matrix, 180-mm field of view (FOV), first-order motion compensation, gradient spoiling, and superior presaturation slab. Thus, a spatial resolution of 0.70 × 0.94 × 1 mm and an acquisition time of 5 minutes 30 seconds were achieved. Angiographic images were reconstructed using a maximum intensity projection (MIP) algorithm. Two sets of 48 MIP images were generated around head-to-foot and right-to-left axes for a total of 360°. Two hard-copy films of 12 selected MIP images (15° increments for a total of 180° around each axis) were printed for interpretation. The individual native slices were not available for the reviewing process.
IA-DSA
Selective IA-DSA studies of intracranial vessels included at least two orthogonal views of each artery with additional oblique projections if necessary. The images were obtained with a 0.6-mm focal spot and displayed with a 512 × 512 matrix.
Selection of MR Angiographic and IA-DSA Studies for Comparison
Forty-seven IA-DSA studies performed within 24 hours of MR angiography were available for comparison. The mean delay between IA-DSA and SAH was 10.6 ± 5.4 days. Thirty-four IA-DSA studies were performed within 8 hours of MR angiography. A maximum delay of 24 hours between IA-DSA and MR angiography was tolerated if the examinations were performed in asymptomatic patients at a time remote from the spasm period (two examinations at day 0 and seven examinations more than 3 weeks after SAH) or if symptoms and transcranial Doppler sonography indicated that the intracranial vascular status was stable within this period and that no change in therapy occurred between MR angiography and IA-DSA (three examinations). For 77% of the comparisons, MR angiography was obtained before IA-DSA.
Spasm Evaluation
MR angiographic and IA-DSA studies were visually assessed by four neuroradiologists (two raters for each technique) who followed the same evaluation protocol for determining vascular narrowing. The reviewers were blinded to the patients' clinical condition and to the results of the other technique. They independently evaluated the right and left ICA, ACA, and MCA and then formed a consensus for all vessels. Vascular narrowing was rated on a four-point scale with the following criteria: 0 = no narrowing, 1 = slight narrowing (<25% reduction in lumen diameter), 2 = moderate narrowing (25–50% stenosis or 50–75% stenosis affecting only a short segment of vessel), and 3 = severe narrowing (50–75% stenosis affecting a long segment of vessel or any stenosis >75%). In addition, the reviewers indicated whether vessel narrowing was presumably due to a spasm or to a preexisting condition, such as developmental hypoplasia, atheromatosis, or vasculitis. The proximal ACA (A1) segment was considered hypoplastic if the contralateral A1 segment and the AComA were large and if the A2 segments were both well filled. Confidence in identifying A1 hypoplasia was increased if no spasm was present in any other arteries, especially when the aneurysm was not on the AComA. The analysis was performed twice. First, the 47 MR angiograms obtained within 24 hours of IA-DSA were evaluated alone (analysis A). Second, all MR angiograms obtained in the same patient were given to the reviewers, who then rated each study, including the examination that had already been evaluated (analysis B). With this second rating, we tested the hypothesis that comparing several MR angiograms obtained in the same patient (real clinical situation) would increase the reading accuracy by revealing the chronological evolution of vessel narrowing, helping to differentiate artifacts from actual narrowing and spasm from hypoplasia. In this second evaluation, the consistency of A1 segment narrowing throughout all MR angiographic examinations (including those performed at a time remote from the vasospasm period) was considered an additional criterion of hypoplasia. The quality of all MR angiograms was evaluated and a score was assigned to each examination: 0 = good quality, 1 = moderate artifacts, and 2 = severe artifacts. The presence of subarachnoid methemoglobin, appearing as hyperintense areas surrounding the vessels on the MIP reconstructions, was also recorded, and the reviewers indicated whether this hyperintensity was a limiting factor in delineating the vessels.
Statistical Analysis
Agreement between MR angiography and IA-DSA was assessed with weighted κ statistics calculated on the 47 pairs of examinations. The comparison was made for all rated vessels together (six vessels per patient) and then separately for the ICA, the ACA, and the MCA (two vessels per patient for each location).
Sensitivity, specificity, accuracy, and positive and negative predictive values were also calculated. For this purpose, the vessel narrowing scores were grouped into two categories: 0 and 1 = no angiographic spasm and 2 and 3 = angiographic spasm (except if the reviewers indicated that this narrowing was not related to a spasm but to hypoplasia or preexisting stenosis). The standard of reference for identifying vessel narrowing was the IA-DSA study. However, MR angiograms obtained at a time remote from the spasm period were used as references for differentiating hypoplasia from spasm to correct for potential errors in IA-DSA interpretation. Sensitivity, specificity, accuracy, and positive and negative predictive values were calculated 1) for each location (right and left together, two vessels per patient for each location) and 2) for each patient (a spasm was considered present if at least one vessel was spastic). All statistical tests were performed twice on the 47 MR angiograms and IA-DSA studies that were compared: once with the results of analysis A and once with the results of analysis B.
Results
Overall Interpretation of the 149 MR Angiograms
The MR examination was well tolerated by all patients. Intramuscular sedatives were administered before the examination only in a few agitated patients (11% of all MR angiographic studies). Images were of good quality in 123 cases (83%), had moderate artifacts in 20 cases (13%), and had severe artifacts in six cases (4%). The presence of methemoglobin was recorded on 24 MR angiograms (16%), but these bright areas were a limiting factor for vessel delineation only in six cases. By performing systematic MR angiography in patients with acute SAH, we were able to detect angiographic vasospasm in 20 of 42 patients.
Comparison between MR Angiography and IA-DSA
Table 1 summarizes the results of the comparison between MR angiography and IA-DSA made with κ statistics. Overall, the agreement between MR angiography and IA-DSA was substantial (κ = .70 in analysis A and κ = .72 in analysis B). When the results were sorted by location, the agreement remained substantial for the MCA and ACA in both groups. For the ICA, the agreement was only moderate (κ = .50) in analysis A but was substantial (κ = .61) in analysis B.
Weighted {κ} statistics showing the agreement between MR angiography and IA-DSA in detecting vasospasm
At IA-DSA, angiographic vasospasm was seen in 12 (26%) of 47 studies. In this study population, 31 of 282 vessels showed an angiographic spasm (eight ICAs, 14 ACAs, nine MCAs). The number of true- and false-positive findings and true- and false-negative findings on MR angiography as well as the sensitivity, specificity, accuracy, and positive and negative predictive values of MR angiography for diagnosing the presence of angiographic vasospasm are recorded in Table 2. MR angiography detected 92% of patients with angiographic vasospasms. The specificity of MR angiography was 97% in analysis A and 94% in analysis B. This led to a high degree of accuracy (96% in analysis A and 94% in analysis B) and to high positive and negative predictive values. The specificity was high for individual vessel assessment (91–99%). The sensitivity was excellent for the ACA (100%) but was poorer for the ICA and MCA. For the ICA, the availability of other MR angiograms obtained in the same patient increased the sensitivity of MR angiography from 25% (analysis A) to 50% (analysis B). Because the prevalence of vasospasm for individual vessels was low, the positive predictive values were moderately good (67–83%), but the negative predictive values were very high (93–100%) and the accuracy was very good (93–96%).
Results of MR angiography in detecting angiographic vasospasm
Discussion
This study shows that serial MR angiography is accurate in the detection of vasospasm in patients with acute spontaneous SAH. Overall, the agreement with IA-DSA was substantial and the sensitivity and specificity of MR angiography for identifying patients with vasospasm were 92% and 97%, respectively. When each vessel was considered independently, the specificity for all locations and the sensitivity for the ACA were excellent, although sensitivity for the ICA and MCA was poorer.
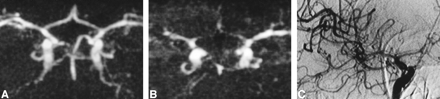
Serial MR examinations performed in patients with acute SAH may be considered controversial (17–18). However, even though MR angiography does not offer the facility of a bedside method, such as transcranial Doppler sonography, our study shows that it is possible to use this technique to detect and follow up vasospasm, because the method is fast, well tolerated, noninvasive, and can be performed even in critically ill patients thanks to the modern magnetic-compatible monitoring devices (20). The majority of the MR angiograms were of good quality. In the case of diffuse vasospasm, arterial narrowing and slow flow typically resulted in a poorly resolved angiogram that should not be confused with artifacts or technical problems (Fig 1).
A and B, MR angiograms (32/8/2; flip angle = 17°) obtained 1 (A) and 10 (B) days after acute SAH. At day 1, all vessels are normal (rating = 0); at day 10, a severe vasospasm is identified in both the ACA (rating = 3 bilaterally) and in the right MCA (rating = 3). In the left MCA, the narrowing is moderate (rating = 2) and a slight narrowing of both ICAs was also recorded (rating = 1 bilaterally) (not shown). The distal segments of arteries are poorly visualized, and the overall quality of MR angiography looks poor. These findings are typical of severe vasospasm and are related to the decreased flow into spastic vessels.
C, IA-DSA (right carotid arteriogram) obtained 9 days after SAH and 15 hours before MR angiography (B). Note presence of severe vasospasm (rating = 3 for the ACA and 2 for the MCA and ICA). Correlation with MR angiography was excellent for the ACA and good for the MCA and ICA.
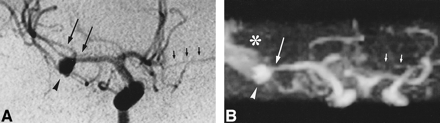
With a TOF technique, the presence of methemoglobin in subarachnoid spaces reduces the T1 of CSF, which may result in irregular hyperintense areas that are recognized by MIP processing. This hyperintense signal is therefore superimposed on the MR angiogram, decreasing the contrast between vessels and surrounding background and making vessel delineation more difficult (18) (Fig 2). Phase-contrast MR angiography could easily overcome this problem, but the acquisition time is much longer for the same spatial resolution and it has been shown that 3D phase-contrast MR angiography is less reliable in grading intracranial vascular stenosis than is 3D-TOF MR angiography (21). As methemoglobin was a limiting factor in only 4% of the 3D-TOF MR angiograms, we do not advise the use of 3D phase-contrast MR angiography for routine screening of vasospasm.
A, IA-DSA (right carotid arteriogram) obtained 8 days after SAH. An 8-mm aneurysm (arrowhead) is apparent in the right MCA and a slight vasospasm is seen in the MCA near the aneurysm (long arrows; rating = 1). The left ACA (A1 segment) is hypoplastic (short arrows).
B, MR angiogram (32/8/2; flip angle = 17°) obtained 3 hours before IA-DSA. The presence of methemoglobin (asterisk) decreases the image quality but interpretation is still possible. Only the distal segments beyond the right MCA trifurcation cannot be delineated owing to the superimposition of hyperintense signal arising from methemoglobin. The aneurysm is clearly identified (arrowhead) as well as the narrowing of the right MCA (long arrow; rating = 1) and the hypoplasia of the left ACA (short arrows). Correlation with IA-DSA appearance is excellent.
The major drawback of our study was the use of a 0.5-T magnet and an MR angiography protocol that did not include recent technological improvements (19). Because our patients had all suffered an SAH, we wanted to keep the examination time as short as possible. This was achieved by reducing the FOV to the area around the circle of Willis. This limited FOV did not allow depiction of peripheral arterial segments and limited our work to the anterior circulation, as the posterior circulation was not reliably included on all MR angiograms. We have previously shown that a 0.5-T system is not a limitation for detecting aneurysms with MR angiography (20), and our present study demonstrated that, even with a suboptimal technique, high diagnostic accuracy can be achieved in identifying patients with vasospasm. However, it is expected that an up-to-date 1.5-T system, improved signal-to-noise ratio, better spatial resolution, and a larger FOV will contribute to even better performance.
The agreement between MR angiography and IA-DSA was substantial except in the ICA when MR angiograms were read alone without the benefit of follow-up studies (analysis A). It is well known that MR angiography tends to overestimate vascular stenosis because of the signal loss created by turbulence and acceleration of flow (18). However, experienced interpreters are aware of this technical bias and can mentally counterbalance these effects when grading a stenosis by assigning a percentage of stenosis that is less than that actually seen on images. Eventually, this may lead to an underestimation of stenosis (22). In our results, we observed more overestimations (30/282) than underestimations (22/282) of vessel narrowing with MR angiography in the cases of disagreement between MR angiography and IA-DSA. The disagreements occurred mainly between categories 0 and 1 or 2 and 3, which may reflect the difficulty in visually assessing the exact degree of stenosis on MR angiograms and in appreciating the subtle differences between categories 0 and 1 and 2 and 3. When the results were grouped into only two categories (no angiographic spasm and angiographic spasm), MR angiography did not overestimate the presence of spasm.
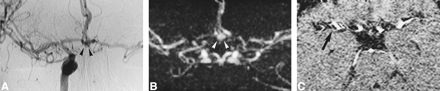
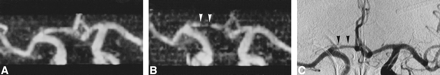
The specificity of MR angiography for detecting vasospasm was very good for all vessels; sensitivity was excellent for the ACA but was lower for the ICA and MCA. The difficulty in evaluating the intracranial portion of the ICA is not surprising, because substantial flow and susceptibility artifacts are present in the region of the carotid siphon (18, 20). The rather poor sensitivity in detecting vasospasm in the MCA was less expected but can also be explained by the tortuous vessel anatomy of the MCA bi- or trifurcation, the artifacts related to subject motion, or the pulsatile vessels, which are more frequently projected into the region of the MCA in this particular patient population. The small FOV of MR angiography, which included only a short portion of the M2 segments, was also a limiting factor in appreciating diffuse peripheral spasm. Figure 3 illustrates vessel pulsatility that can lead to a false diagnosis of vasospasm on MIP images. This misinterpretation can be avoided by looking at the native slices in every case of poorly visualized vessels; however, the reviewers in this study interpreted only the MIP angiogram, and individual partitions were not available. Our hypothesis that a comparison of several MR angiograms obtained in the same patient would improve the accuracy of MR angiography in detecting vasospasm was not confirmed. The detection of patients with angiographic spasm did not differ when the MR angiogram was rated alone or together with all follow-up studies. However, we were able to show an increase in sensitivity for detecting vasospasm in the ICA in analysis B. In the carotid siphon, the artifacts are expected to remain the same from one examination to another, and a change of vessel diameter can be more easily interpreted as vasospasm when a series of MR angiograms is available. Both reviewers also agreed that their confidence was much higher when several MR angiograms were available, and this might be helpful for less experienced readers. An example of the utility of serial MR angiograms in differentiating A1 hypoplasia from A1 vasospasm is shown in Figure 4. Our results appear better than those published by Tamatani et al (23), but substantial differences exist in the methods and in the patient populations between the two studies. In their series, the presence of artifacts created by aneurysm clips interfered with the diagnosis of vasospasm. Almost all our MR angiograms were obtained before the clipping operation. However, the recent use of clips made of titanium combined with short-TE MR sequences considerably reduces these artifacts (24). It is also unlikely that a clinically significant vasospasm would be limited only to the short arterial segment immediately adjacent to the clip without any narrowing of slightly remote segments. MR angiographic evaluation of vasospasm should therefore be possible even in the presence of surgical clips, but further studies are needed in these conditions.
A, IA-DSA (right carotid arteriogram) obtained 16 days after acute SAH. A 5-mm aneurysm is visible in the AComA (arrowheads). There is a slight narrowing of the ICA (rating = 1), but overall there is no angiographic vasospasm.
B and C, MR angiograms (32/8/2; flip angle = 17°) obtained 2 hours before IA-DSA. The aneurysm is clearly visible (arrowheads). On the MIP image (B), some portions of arteries are poorly visualized on both sides, suggesting the presence of a significant vasospasm (rating = 2 for both ICA and ACA and 1 for both MCAs). This is a false interpretation, which can be avoided by looking at the native slices (C). Significant pulsation artifacts (arrow) are present around the arteries, leading to a discontinuous aspect of vessels on the MIP image.
A and B, MR angiograms (32/8/2; flip angle = 17°) obtained 2 (A) and 9 (B) days after acute SAH. At day 2, all vessels are normal (rating = 0); at day 9, significant vasospasm of the right ACA is identified (A1 segment, rating = 2) (arrowheads, B). The diagnosis is easy when a previous MR angiogram is available for comparison.
C, IA-DSA (right carotid arteriogram) obtained 10 days after SAH and 18 hours after MR angiography (B). The narrowing of the right A1 segment (arrowheads; rating = 2) was thought to be hypoplasia by one of the reviewers, but MR angiography performed before the spasm proved this interpretation to be incorrect.
We did not correlate the results of MR angiography with the patients' clinical scores. Our purpose was only to evaluate the accuracy of MR angiography as compared with IA-DSA in detecting vessel narrowing due to vasospasm. It is well known that vasospasm identified at angiography does not necessarily correlate with clinical symptomatology and that, in some patients, angiographic vasospasm may be asymptomatic (25). Some compensation mechanisms (including local vasodilatation and collateral flow) may allow a preservation of blood supply and metabolism. It is not expected that the relationship between angiographic spasm and clinical symptoms would be different whether vessel narrowing is identified at MR angiography or IA-DSA.
It is premature to define how MR angiography could be integrated into the evaluation of vasospasm in patients with SAH, but the feasibility and the accuracy of MR angiography in this setting suggest that it might play a role alongside transcranial Doppler sonography and IA-DSA. Transcranial Doppler sonography is quite good for screening vasospasm, but it may be limited by a poor acoustic window and by its inability to address distal segments. It is excellent for diagnosing vasospasm of the MCA (M1 segment), but remains a poor tool for identifying spasm in the ICA and ACA, leading to an overall sensitivity of 70% (11). Moreover, the relationship between the measured velocities and the importance of vasospasm may be altered by triple H therapy, making transcranial Doppler sonography suboptimal for evaluating treatment efficacy. Compared with transcranial Doppler sonography, MR angiography is not limited by acoustic windows, has the potential to depict more distal arterial segments, and is unbiased by the treatment. Nevertheless, its performance is inferior to that of IA-DSA, especially for depicting vasospasm in the ICA and MCA, and for quantifying the exact degree of vessel narrowing. MR angiography could be considered a useful complement to transcranial Doppler sonography, especially for diagnosing ACA vasospasm, where its performance is excellent, and when transcranial Doppler sonography is impossible or its interpretation questionable. In these cases, diagnostic IA-DSA is still performed in many centers and is not necessarily followed by interventional procedures (11). MR angiography may help to restrict the use of IA-DSA to only those patients who do not respond to medical treatment and in whom interventional procedures are planned.
If visualization of the arterial tree is useful for choosing the most appropriate treatment, the ultimate finding for identifying tissue at risk might be a local decrease in CBF. In patients treated with hypervolemia/hemodilution, it has been shown with xenon-enhanced CT that the topography of locally decreased CBF corresponds to the neurologic deficit. Inversely, increased flow velocity recorded at transcranial Doppler sonography does not correlate with neurologic findings and does not necessarily correspond to decreased CBF, although it could be associated with hyperemia (14). Decisions regarding management of patients with SAH should therefore be based on both an anatomic angiographic technique and a functional technique measuring CBF (5, 14). MR imaging has the potential to explore both these aspects in a unique, comprehensive examination. A recent pilot study in six patients has shown the usefulness of diffusion-weighted and perfusion-weighted MR imaging in patients with acute SAH (26). Further studies should determine whether MR angiography combined with these techniques could become a useful tool in the clinical management of patients with acute SAH.
Conclusion
Our study has shown that MR angiography at 0.5 T is feasible and accurate for identifying patients with angiographic vasospasm after acute SAH but is less sensitive than IA-DSA for depicting vasospasm of the ICA and MCA. Further studies combining state-of-the-art MR angiography and functional MR imaging in patients at risk for vasospasm should help to define the role of MR imaging and MR angiography in the clinical management of patients with SAH.
Footnotes
↵1 Address reprint requests to Cécile B. Grandin, MD, Department of Medical Imaging (MRI Unit), 10 Hippocrate Ave, B-1200 Brussels, Belgium.
References
- Received July 19, 1999.
- Accepted after revision April 19, 2000.
- Copyright © American Society of Neuroradiology