Abstract
Summary: A 13-month-old boy developed eosinophilic meningoencephalitis, retinitis, and a protracted encephalopathy with severe residual deficits. The initial MR examination revealed diffuse periventricular white matter disease, and follow-up images showed atrophy. Brain biopsy, serology, and epidemiologic studies lead to the diagnosis of Baylisascaris procyonis infection, a parasitic disease contracted through exposure to soil contaminated by the eggs of a common raccoon intestinal roundworm. The pathologic, epidemiologic, and imaging features of this disease are herein reviewed.
Raccoons are widely distributed throughout the United States and frequently reside in heavily populated urban or suburban areas. They are commonly infected with Baylisascaris procyonis, an intestinal roundworm known to cause neurologic disease (neural larva migrans) in a variety of animals, including humans (1−7). We encountered a suburban toddler with an acute illness characterized by eosinophilia, white matter disease, and retinitis caused by B. procyonis infection. This is the fourth such case reported, likely acquired by oral ingestion of soil contaminated with raccoon feces. This case is notable for its neuroradiologic and pathologic findings, which are presented herein to highlight the clinical entity of raccoon roundworm encephalitis.
Case Report
This 13-month-old boy was well until he developed listlessness and right esotropia. There had been no fever or obvious antecedent illness, and he had had no known medical problems. He was seen by his pediatrician the next day, but a physical examination was unrevealing. During the next 2 days, he developed increasing lethargy, bilateral esotropia, and deterioration in speech and fine motor tasks. Three days after onset, he was noted to stagger and had to support himself with his arm while seated. MR imaging performed at presentation 4 days after onset revealed bilateral, diffuse white matter abnormalities in the deep cerebellum and periventricular regions (Fig 1A).
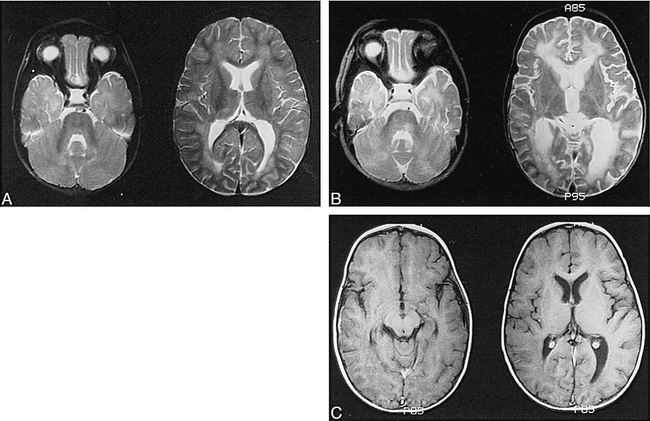
Images from the case of a 13-month-old boy who was well until he developed listlessness and right esotropia.
A, MR image obtained at presentation. Bilateral, patchy T2 hyperintensity is seen predominantly in the white matter, including the periventricular regions and corpus medullaris of the cerebellum. Poor gray-white matter distinction and a suggestion of cortical edema are also seen, particularly in the left temporoparietal region.
B, MR image obtained at 6 weeks. A marked progression of white matter changes can be seen more extensively throughout the supratentorial regions and in the tegmentum of the brain stem. Note interval enlargement of the ventricles and sulci, consistent with atrophy.
C, Follow-up T1-weighted images obtained after the IV administration of contrast material show no abnormal enhancement of the meninges or parenchyma.
The patient was admitted to the hospital 5 days after onset, and the results of a general physical examination were remarkable for a temperature of 99.6°F and a coarse macular rash on the face and ear. The results of a neurologic examination performed at the time of admission included poor tone in the legs but were otherwise unremarkable. An ocular examination revealed a normal left fundus. The right eye had a diffusely mottled appearing retina, a reddish lesion of the macula, and a suggestion of optic pallor. Visual evoked potentials were entirely absent on the right. Although the patient's gaze appeared dysconjugate at times, no reproducible defects in ocular motility were found.
Laboratory studies included a peripheral WBC count of 28,400 mm3, with 45% eosinophils, 24% neutrophils, 29% lymphocytes, and 2% monocytes. The remaining results of the routine blood work were normal. A lumbar puncture performed 6 days after onset yielded clear, colorless fluid with 0 RBC, 1 WBC, glucose of 65 mg/dL, and slightly elevated protein (54 mmol/L; upper limit of normal for lab = 45 mmol/L). The CSF sediment was notable for prominent eosinophils, with lymphocytes and monocytes. CSF cultures, stains for acid fast bacteria, assays for herpes simplex I and II, coccidiomycosis titers, and the results of the Venereal Disease Research Laboratories test were all negative or normal. A chest X-ray showed mild bihilar and right middle lobe infiltrates. The results of skin tests were negative for coccidiomycosis, diphtheria, and candida. The results of a stool examination for ova and parasites and serologies for Toxocara and Leptospira were negative. An extensive metabolic workup yielded normal results, except for elevation of serum lactate. The electroencephalographic results were also normal.
Leading differential considerations at the time of admission included acute disseminated encephalomyelitis and visceral larva migrans owing to either Toxocara or Baylisascaris. Toxocara was thought to be unlikely because of negative serologic findings and the presence of prominent brain involvement. Baylisascaris serologic analysis was unavailable at the time. Acute serum was banked, and the child was empirically treated with steroids (Solumedrol and prednisolone), antibiotics (Cefotaximine, streptomycin, rifampin, isonicotinic acid hydrazide, and pyrazinamide), acyclovir, and B6. The peripheral eosinophils diminished, and the rash and pulmonary infiltrates cleared. The patient's neurologic disease, however, worsened slowly over months, with impaired vision and a right hemiparesis. Follow-up MR images showed progression of disease, with increasingly confluent white matter changes, evidence of diffuse atrophy, and new involvement of the pontine tegmentum and bilateral insular regions (Fig 1B). There was no evidence of abnormal meningeal or parenchymal enhancement after the IV administration of contrast material on images from two separate MR imaging studies (conducted 12 and 31 days after onset), but the images in both studies were obtained after the administration of steroids was begun (Fig 1C).
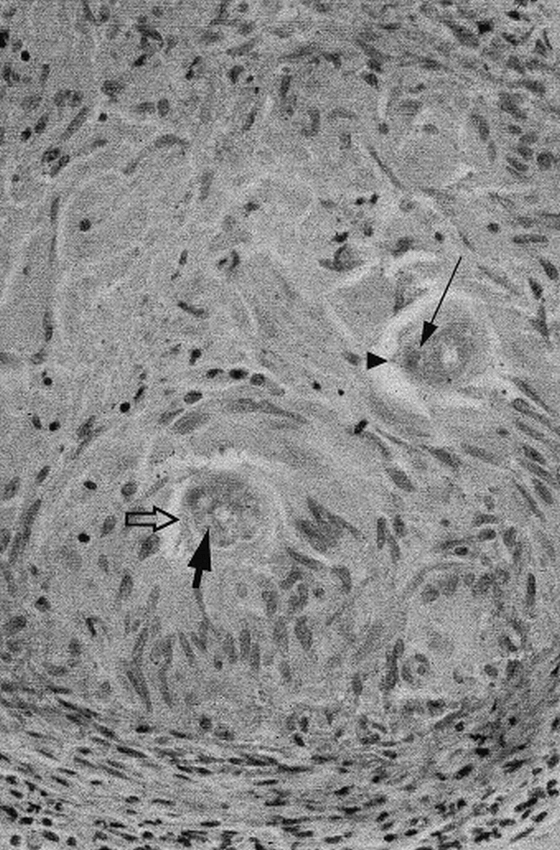
Muscle and brain biopsies were performed approximately 11 months after onset. Sections of skeletal muscle displayed Type I muscle fiber predominance but were otherwise normal. Sections of left frontal cortex and subcortical white matter revealed scattered macrophages and aggregates of lymphocytes. One fragment of tissue displayed a single large granuloma (400 μm in diameter), which contained two cross sections of a nematode larva (Fig 2). The larva measured 36 to 40 μm in diameter and had prominent lateral alae (longitudinal ridge-like structures), a large, centrally located intestine with prominent intestinal granules, and paired, triangular-shaped lateral excretory columns. The overall larval size, shape, and proportion of excretory columns and intestine and other characteristics matched those of Baylisascaris larvae and not other known causes of larva migrans (2, 7). The size and features of the larva indicated that the sections were from the posterior intestinal region, not the larger midbody area. The sections matched similar sections from proven cases of B. procyonis encephalitis in animals.
Baylisascaris procyonis: larval granuloma (400-μm diameter) in biopsy of cerebral cortex. Note cuticle (hollow arrow), lateral ala (arrowhead, ridge-like structure), intestine (thick filled arrow), and excretory columns of the parasite (thin arrow)
The child lived in San Leandro in the suburban San Francisco Bay area of northern California. The household included two cats and a dog. A health department inspection of the home revealed what appeared to be dog and cat feces in the yard and in the child's sandbox. Although the parents were unaware of their child being exposed to wild animals, opossums had been seen in the neighborhood and raccoons were very common. A subsequent investigation (L. Frazer, Santa Clara County Vector Control, and W. Murray, San Jose State University) indicated that at the time of the child's illness, the neighborhood was having a significant problem with raccoons. Raccoons were commonly seen, both day and night, in a large fig tree and grape arbor in the child's backyard. San Leandro Animal Control records listed numerous calls reporting the nuisance from raccoons. A woodpile was also present nearby, a site known to be conducive to raccoon fecal contamination (1, 8). Because of ongoing raccoon problems, neighbors trapped and removed more than 30 raccoons from the vicinity. Several studies have indicated the high prevalence of B. procyonis in raccoons in the area and that raccoon fecal contamination commonly occurs in residential yards (7, 9) (W. Murray, San Jose State University [unpublished data]). Based on the large number of raccoons seen in the patient's yard at the time of his illness, exposure to contaminated soil on the premises was considered the likely source of his infection with B. procyonis.
Subsequent examination of banked acute phase serum by enzyme-linked immunosorbent assay, performed using B. procyonis larval excretory-secretory antigens, was strongly positive for antibodies to B. procyonis at a titer of approximately 1:102,400. Serum also gave strong positive reactions to B. procyonis third-stage larvae by indirect immunofluorescence, with a titer of 1:1024 and good differential staining of sections (K. R. Kazacos, Purdue University).
Follow-up
At 7 months' follow-up, the patient's course had stabilized. He then developed seizures, which remained medically refractory. Follow-up MR imaging showed partial resolution of white matter signal changes, but diffuse atrophy persisted. Occasional mild peripheral eosinophilia was seen at up to 6%. At 7 years' follow-up, the patient's condition had stabilized and there were severe residual deficits. Although he was able to feed himself and respond to his mother's voice, he was functionally blind, was incontinent, uttered only a few syllables, and was largely confined to wheelchair use. He suffered two to three myoclonic seizures per day, despite multiple anticonvulsants. He received synthetic thyroid hormone replacement for hypothyroidism. Two siblings, one older and one younger than the patient, remained normal.
Discussion
Based on pathologic larval identification, strongly positive serology, compatible clinical disease, and probable exposure, we concluded that this child was infected with larvae of B. procyonis, the common raccoon ascarid. CNS infection by B. procyonis can cause severe morbidity and mortality in humans and other animals (1−7). It has been documented as a cause of pediatric encephalitis in three previous cases (3−5), two of which were fatal (Table). This is the fourth reported case of proven human brain infection, the first in the western United States. Six additional cases of fatal or severe CNS infection in children are also known, further indicating the importance of this disease (K. R. Kazacos [unpublished data]). B. procyonis is the most commonly recognized cause of clinical larva migrans in animals, having produced CNS disease in more than 90 species of mammals and birds (1, 2) (K. R. Kazacos [unpublished data]). Knowledge of the pathogenesis of B. procyonis and of the neuroimaging characteristics of B. procyonis encephalitis is important in the early diagnosis and treatment of this infection.
Imaging features of human Baylisascaris encephalitis
Epidemiology and Pathogenesis of Infection
B. procyonis is endemic in raccoons in most regions of the United States, with a prevalence of 68% to 82% in the midwest, in the northeast, and on the west coast (1, 7, 9). The risk to humans occurs when B. procyonis eggs are ingested directly from raccoon feces or from areas or articles contaminated by them (eg, logs, favorite wood piles, associated soil, and barn lofts that raccoons use as communal toilets) (1, 8, 10). Because of the high concentration of infective eggs associated with raccoon toilets, these areas are important sources of infection for humans and animals (1−5, 8, 10). An infected raccoon can shed millions of B. procyonis eggs each day, and these eggs are very resistant to environmental degradation, lasting several years in the soil (given adequate moisture) (1). In northern California, several studies have found two thirds or more of raccoons to be infected (7, 9) (W. Murray [unpublished data]). Infection and clinical disease have been described in a variety of domestic and zoo animals that also became infected from environmental sources. It becomes clear that preventative measures designed to limit the ingestion of infective eggs are of paramount importance, especially for children.
After the ingestion of infective eggs, Baylisascaris larvae undergo aggressive migration and enter a variety of somatic tissues, including the CNS and eyes. The larvae grow considerably during migration and give off an array of enzymes, waste products, and cuticular components (1, 2). Larval migration causes tissue damage, necrosis, and a marked inflammatory reaction, of which eosinophils are a major component. It has been suggested that toxic eosinophil proteins released into the brain and other tissues contribute to the pathologic changes and clinical signs seen with this infection (1, 2). Clinical CNS disease is related to the number of larvae entering the brain and to the severity of CNS damage and inflammation.
Clinical Spectrum and Treatment of Human Disease
B. procyonis infection affects both children and adults, usually as an encephalitis, a neuroretinitis, or a combination of the two. All three of the previously documented cases in the United States and the present case occurred in young children presenting with signs and symptoms of severe encephalitis (3−5); one child also had retinal abnormalities (3). A presumptive case of B. procyonis encephalitis in an adult was mentioned in one case report (5), and death of a 10-year-old resulting from eosinophilic cardiac granuloma was ascribed to B. procyonis (11). A number of cases in adults have manifested as unilateral ocular infection without accompanying CNS signs (2, 6, 7, 12). In these cases, the moving worms seen on the retina were directly photocoagulated. Late imaging of our patient showed slightly atrophic-appearing optic nerves, but the degree of tissue loss was proportionate to that seen in the brain. Blindness has been a common feature, even when CT or MR imaging reveals no obvious cortical damage and retinal examination findings are normal. Specific visual pathway involvement may represent CNS pathologic or functional changes that are difficult to detect with imaging techniques or a simultaneous neuroretinitis or optic neuritis may account for these findings. Our patient had evidence of both diffuse brain disease and focal retinal infection. Considering the widely distributed involvement throughout the brain and retina, it is presumed that the infection involved multiple B. procyonis larvae.
The course of the disease varies widely. The parasite is known to cause asymptomatic, mild or severe clinical disease referable to the CNS or eyes (1−7). Some patients remain asymptomatic despite seropositivity (5), which is probably a common outcome of infection (2). Others have a protracted course with residual dysfunction (5), as in the present case. Young children infected with large numbers of eggs succumb to overwhelming disease (3, 4). This spectrum of disease correlates with the dose of eggs ingested as well as the degree of CNS migratory damage. Young children or mentally impaired adults with pica are more likely to directly ingest feces or fecally contaminated material containing eggs than are older children or unimpaired adults. Infants are at the greatest risk of heavy infection (1, 2). Although one previous case occurred in a child with Down syndrome (4), which may have predisposed to pica, the underlying diagnosis was probably otherwise unrelated. There have been no reports of obvious immune deficiency to explain any underlying predilection for infection with B. procyonis.
A complicating factor in this infection is the lag time in onset of clinical signs after infection, which results in delayed diagnosis and therapy. Because considerable CNS damage often occurs by the time a diagnosis is made, in most cases, antihelminthic and other therapies are at best stabilizing, and the patient later develops postinflammatory CNS atrophy, as was seen in the present case and others (Table). Recent findings in animals, however, suggest that high doses of albendazole administered early in the course of infection may halt the further development of CNS disease (13, 14) (S. Barrett [unpublished data]). It may also be of benefit in mild cases of CNS infection. Steroids are of definite benefit in most cases through their reduction of CNS inflammation. To institute proper treatment, early tentative diagnosis through clinical signs, exposure history, serologic analysis, and imaging findings is critical.
Neuroradiology
Neuroradiologic abnormalities are common in B. procyonis encephalitis, most often showing diffuse T2 prolongation of the cerebral white matter with a predilection for a periventricular distribution (Table 1). A 17-year-old gibbon with B. procyonis, however, had a single focal lesion in the corona radiata revealed by MR imaging, indicating that imaging findings may be more localized (15). Our case showed progressive deep, periventricular T2 prolongation as well as capsular and brain stem changes, later accompanied by global atrophy. Diffuse white matter disease is a nonspecific imaging finding observed in many children with a variety of inherited and acquired disorders (16). The differential considerations in a given case will depend on the clinical course, exact pattern of white matter disease (deep versus peripheral, focal versus diffuse), and other associated factors. The neuroradiologic analysis must consider the many causes of diffuse white matter disease, with the differential diagnosis narrowed by the clinical context of CSF eosinophilia.
In a previously healthy child, an acute illness accompanied by white matter changes should first raise the possibility of acute disseminated encephalomyelitis. The lesions of acute disseminated encephalomyelitis tend to be patchy and commonly involve both deep gray and white matter. Acute disseminated encephalomyelitis characteristically shows a more multifocal, discrete pattern of lesions than the diffuse disease shown in most cases of B. procyonis encephalitis. It is also monophasic and nonprogressive, helping to distinguish it from the present case. Demyelinating disease caused by multiple sclerosis shows a periventricular predilection, but such plaques are typically more sharply demarcated than the lesions of B. procyonis. Multiple sclerosis is also extremely rare in infants, and none of the above are associated with eosinophilia.
Inborn errors of metabolism and exposure to some toxins may mimic the diffuse white matter disease and later atrophy shown in our case. Some disorders such as organic acidemias may first become clinically evident during an unrelated acute infection, such as a cold or flu, potentially confusing the clinical picture. Urea cycle disorders, nonketotic hyperglycinemia, peroxisomal disorders, maple syrup urine disease, and the effects of previous radiation or chemotherapy could mimic imaging aspects of the current case but would be distinguished by the clinical associations and lack of eosinophilia. Specific brain stem tract involvement seen with adrenoleukodystrophy and tegmental changes seen with maple syrup urine disease are typically symmetric, not patchy or unilateral as shown in our case. White matter involvement from Canavan's or Alexander's disease could usually be distinguished by abnormal early development and association with an enlarged head circumference. Toxin exposure, such as cyanide ingestion or carbon monoxide inhalation, should also be considered as a cause of diffuse white matter disease, but these insults are typically accompanied by basal ganglia involvement. Whether MR spectroscopy could assist in distinguishing some of these conditions from B. procyonis remains to be determined.
Because the imaging differential considerations are numerous and varied, evaluation includes analysis of family and personal histories, screening for inborn errors of metabolism, testing for heavy metals, and CSF examination. In our case, the results of these evaluations were negative except for the finding of CSF pleocytosis and both peripheral and CSF eosinophilia. Although some reported cases of B. procyonis encephalitis have shown enhancement on imaging studies, we did not observe parenchymal or meningeal enhancement on high-quality serial MR images obtained during the acute and subacute phases of the illness. We must conclude that there is a relatively limited blood-brain barrier disruption linked to the pathologic granulomatous response seen in our case or that steroids masked such changes.
Eosinophilic Meningoencephalitis: Differential Considerations
The presence of peripheral and CSF eosinophilia in combination with white matter changes on MR images or signs of a neuroretinitis strongly suggests B. procyonis infection in a patient from the United States or Europe. Both peripheral and CSF eosinophilia have been documented in all reported cases of B. procyonis encephalitis. Eosinophilia is seen in less than 3% of randomly sampled CSF specimens (17). The presence of CSF eosinophilia in a given individual directs attention to a relatively small list of disease entities, particularly those of parasitic and fungal origin (17, 18).
Parasites and fungi associated with CSF eosinophilia vary according to geographic locale and patterns of CNS involvement. In the United States, acute Coccidioides immitis is one of the most common causes of eosinophilic meningitis (19). Brain imaging shows a pattern of intense basilar enhancement, hydrocephalus, and sometimes secondary brain infarction. Patients usually have antibodies to Coccidioides in their CSF. Angiostrongylus cantonensis is a leading cause of eosinophilic meningitis worldwide, particularly in southeast Asia and in the Pacific islands. Although the parasite is neurotropic in rats and humans (18, 20, 21), CT scans show no focal lesions and the infection is generally limited to the meninges. Gnathostoma spinigerum is an intestinal parasite of cats and dogs endemic in southeast Asia, China, and Japan (18, 21), which may lead to a syndrome of myeloencephalitis. Hemorrhagic tracks, focal hemorrhages, and involvement of the spinal cord are helpful distinguishing features in these cases, being rare in other causes of eosinophilic meningoencephalitis. Patients also have migrating cutaneous swellings and acute nerve root pain, and the CSF is hemorrhagic or xanthochromic (21).
Other helminthic parasitic infections, including toxocariasis, trichinosis, cysticercosis, schistosomiasis, fascioliasis, and paragonimiasis, have also been linked to eosinophilic meningoencephalitis (18, 21). Toxocara canis larvae are known to invade the CNS of animals but, in contrast to B. procyonis, are not often associated with the clinical CNS disease (2). Similarly, clinically overt brain disease in humans is less frequent with Toxocara, although a few cases of overwhelming infection are known (2, 22−24). In most cases of toxocaral infection, granulomatous lesions and larvae were found in the CNS of patients with unrelated clinical problems or led to subacute symptoms such as headaches and seizures. Imaging studies of CNS toxocariasis show multifocal circumscribed lesions in the brain or a combination of circumscribed and diffuse changes in chronic infections (23, 24). In contrast, B. procyonis has a much greater likelihood of causing severe neurologic disease with significant white matter changes on MR images, as seen in the present case. It is likely that there will be some overlap in the neuroradiologic appearance of the two conditions (eg, in the case of severe toxocaral encephalitis or mild/localized B. procyonis infection, such that results of ancillary diagnostic aids will be important for identification). Toxocara encephalitis patients, usually children with heavy infections, should also have signs of visceral involvement and be positive for toxocaral antibodies in the blood and CSF. Neurocysticercosis, like B. procyonis, may show a spectrum of neural involvement, ranging from an acute encephalitic form to multifocal brain lesions and basilar meningitis. When CSF eosinophilia is seen in these conditions, it is generally less marked, and other clinical/epidemiologic features help eliminate them from consideration. Several noninfectious causes such as Hodgkin's disease, leukemia, drugs, contrast dyes, and idiopathic hypereosinophilia syndrome have also been associated with CSF eosinophilia (17, 18). These entities are not commonly associated with encephalitis or white matter disease, aiding in their differentiation from B. procyonis infection.
Definitive Diagnosis
Immunologic testing for Baylisascaris has become more readily available, aiding in the diagnosis of this infection. Although the diagnosis of Baylisascaris infection can be confirmed by immunologic means (enzyme-linked immunosorbent assay, immunofluorescence, or Western blotting), the definitive diagnosis of B. procyonis still rests on identification of the larvae from tissues taken by biopsy or at autopsy (2). Direct visualization of the worm in the retina may allow a presumptive diagnosis based on size and morphologic features, but the worm is not always seen and the retina may show only nonspecific signs of inflammation (as in the present case). Although more difficult to justify except in severe cases, brain biopsy may be elected; however, the chances of obtaining a portion of worm in the biopsy specimen are extremely small. This is the first reported brain biopsy confirmation of B. procyonis, and we feel fortunate to have obtained diagnostic material from a single sample. The granuloma containing the organism in our case was present at the gray-white junction. In autopsy cases, granulomas bearing larvae have been found in white matter, predominately in the periventricular region (3, 4). If biopsy is elected, the surgeon should consider sampling a deep white matter site.
In summary, patients with B. procyonis encephalitis usually present with a characteristic syndrome of CSF eosinophilia, diffuse white matter disease, and, sometimes, neuroretinitis. Final identification of B. procyonis as the causative agent rests on immunologic analyses, morphologic identification, and epidemiologic findings of probable exposure. Because diagnosis is difficult and treatment is often ineffective, preventative measures are critical. Young children must be monitored for pica/geophagia and prevented from contacting raccoon fecal material or playing in soil that may be contaminated by it. In endemic areas, children should be taught to recognize and avoid raccoon latrines and to wash their hands after playing outside or with animals.
Acknowledgments
The authors thank Drs. M. Walker, B. Berg, and C. Wheeler for valuable early clinical contributions and E. Brown and C. Leininger for help with serology. Special thanks go to L. Frazer and Dr. W. Murray for their epidemiologic investigations.
Footnotes
↵1 Address reprint requests to Howard A. Rowley, MD, University of Wisconsin, Dept of Radiology E3-311, 600 Highland Ave, Madison, WI 53792.
References
- Received June 17, 1999.
- Copyright © American Society of Neuroradiology