Abstract
BACKGROUND AND PURPOSE: Previous studies have shown that clinical localization of trigeminal nerve lesions is inaccurate as compared with MR imaging findings. The purpose of our study was to ascertain the added value of electromyographic (EMG) investigation of the trigeminal nerve reflexes for the improvement of lesion localization and for the preselection of patients for MR imaging.
METHODS: We reviewed the EMG studies of the trigeminal reflexes and the MR imaging studies of 20 patients with unilateral symptoms and signs related to the trigeminal nerve (40 trigeminal nerves examined). The results of the two studies were compared to assess the value of EMG in predicting MR imaging outcome. Lesion localization as demonstrated by EMG was compared with localization at MR imaging. MR imaging was used as the standard of reference.
RESULTS: Eight (40%) of 20 patients had MR imaging findings related to presenting trigeminal symptoms, including five brain stem lesions and three peripheral lesions. Fourteen (70%) of 20 patients had EMG abnormalities related to presenting symptoms and signs. For brain stem lesions, lesion localization as shown by EMG corresponded well with MR imaging findings. EMG yielded a sensitivity of 100%, a specificity of 81%, a positive predictive value of 57%, and a negative predictive value of 100% in predicting MR imaging results. Interobserver agreement was good for both the EMG reflex and MR imaging examinations.
CONCLUSION: Our data suggest that EMG recordings of the trigeminal reflexes can be used to exclude structural lesions in patients with symptoms related to the trigeminal nerve. When a lesion is localized in the brain stem with EMG, a tailored MR imaging examination of this region may be sufficient.
Patients with symptoms related to the trigeminal nerve may present with a broad spectrum of clinical findings, including facial pain, either typical trigeminal neuralgia or atypical pain, numbness, paresthesias, and weakness or trismus of the masticator muscles. At physical examination, impaired pain, touch and temperature sensations, or a decreased or absent corneal reflex may be found (1, 2). MR imaging is considered the imaging method of choice for evaluation of patients with symptoms related to the trigeminal nerve (3, 4). These patients may have lesions anywhere from the brain stem nuclei to the distal extracranial branches (3, 4). The brain stem trigeminal nuclei extend from the upper midbrain to the lower medulla oblongata (1–4). In a previous radiologic study of patients with trigeminal neuropathy, it was suggested that clinical localization of trigeminal nerve lesions was extremely inaccurate as compared with MR imaging findings and that the entire course of the trigeminal nerve should always be visualized (4).
Electromyographic (EMG) investigation of the trigeminal nerve reflexes, including the blink reflex, the masseter inhibitory reflex, and the jaw-jerk reflex, may provide valuable additional information about the site of a lesion that cannot be obtained with physical information (5). When accurate localization of a lesion is possible with EMG, more tailored MR examinations might be possible, limiting MR imaging time. In a previous study of 112 consecutive patients with trigeminal nerve symptoms, 61% of the patients had abnormalities on MR images (6). Preselection of patients on the basis of EMG findings may increase the yield of MR imaging in the evaluation of patients with symptoms and signs related to the trigeminal nerve.
The purpose of our study was to compare the results of EMG examinations of the trigeminal nerve reflexes with MR imaging findings.
Patients and Methods
Patients
Between May 1992 and August 1997, 20 patients (11 men and nine women), aged 25 to 63 years (mean age, 42 years), with unilateral trigeminal nerve symptoms (nine with left-sided and 11 with right-sided symptoms) underwent EMG of the trigeminal reflexes and MR imaging. MR imaging diagnoses were confirmed at surgery and by histopathologic examination, by typical clinical course, or by further radiologic follow-up. Medical records were reviewed to assess type and laterality of presenting symptoms. For patients with negative MR imaging studies, follow-up findings were also evaluated.
EMG
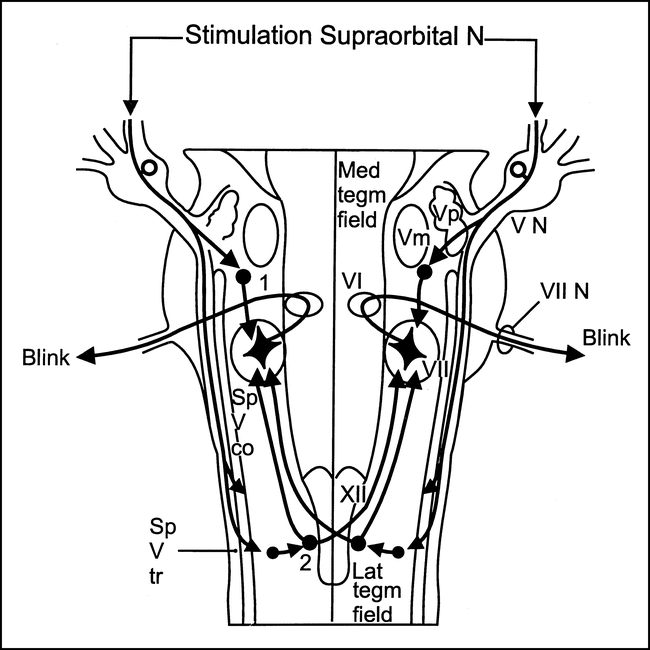
Blink reflex response latencies to supraorbital nerve stimulation on either side were recorded with a fine concentric needle electrode in the orbicularis oculi muscle, according to a technique described previously (7–11). The supraorbital nerve and the sensory ophthalmic root form the common afferent limb; the facial nerve, the common efferent limb. In this reflex arc, there is an early unilateral, R1, response at about 10 milliseconds, relayed through an oligosynaptic pathway through the principal trigeminal nucleus in the middle third of the pons. The later bilateral, R2, responses at about 30 milliseconds are relayed through a more complex pathway in the pons and dorsolateral medulla (7–13) (Fig 1). The afferent fibers for R2 descend from the pons through the spinal trigeminal tract in the medullary region and terminate in the most caudal part of the spinal trigeminal nucleus. From this area, R2 is conducted through ipsilateral and contralateral polysynaptic pathways through the lateral tegmental field before making connections with the facial nuclei (5, 10, 13–15).
Blink reflex. Diagram shows presumed location of the bulbar interneurons subserving the two components of the blink reflex: (1) interneurons subserving the ipsilateral early components; (2) interneurons subserving the bilateral late component. (Vm indicates trigeminal motor nucleus; Sp V co, spinal trigeminal complex; Sp V tr, spinal trigeminal tract; VI, abducens nucleus; VII, facial nucleus; VII, facial nerve; VN, trigeminal sensory root; XII, hypoglossal nucleus; Lat tegm field, lateral tegmental field; Med tegm field, medial tegmental field.) Modified from (5) and used with permission
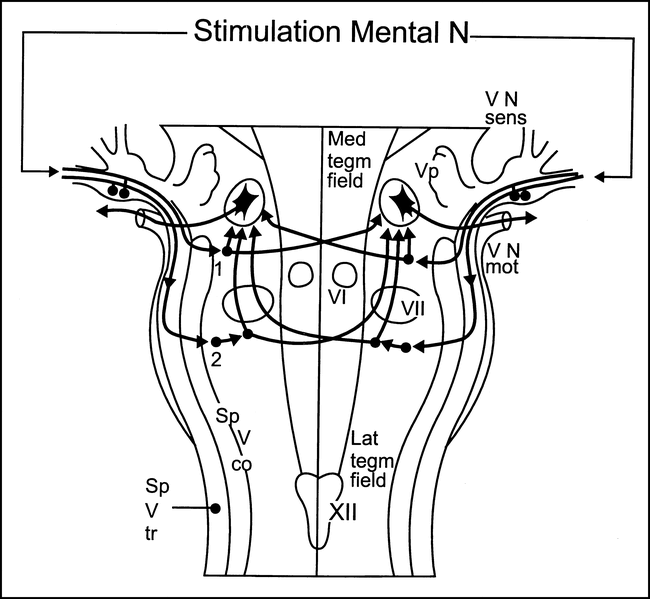
Masseter inhibitory reflex latencies were recorded simultaneously from both masseter muscles by needle or surface electrodes after stimulation of the mental nerve on either side during maximal clenching of the teeth. The masseter inhibitory reflex consists of early symmetrical and late phases of EMG silent periods, with the first silent period (SP1) occurring at 10 to 15 milliseconds and the second silent period (SP2) at 40 to 50 milliseconds, interrupting the voluntary EMG activity in the ipsilateral and contralateral masseter muscles (16, 17). After stimulation of the mental nerve, impulses reach the pons via the sensory mandibular root of the trigeminal nerve. The S1 response is mediated by one inhibitory interneuron projecting onto jaw-closing motoneurons bilaterally. The whole circuit lies in the midpons (5, 16, 17). The afferents for S2 descend in the spinal trigeminal tract and connect with a polysynaptic chain of excitatory interneurons, located in the lateral tegmental field formation, at the level of the pontomedullary junction. The last interneuron of the circuit is inhibitory and gives rise to ipsilateral and contralateral collaterals that ascend medially to the right and left spinal trigeminal complexes, to reach the trigeminal motoneurons (5, 16, 17) (Fig 2).
Masseter inhibitory reflex. Diagram shows presumed location of the bulbar interneurons subserving (1) the early (SP1) phase and (2) the late (SP2) phase of the masseter inhibitory reflex. (VN sens indicates trigeminal sensory root; VN mot, trigeminal motor root; for other abbreviations, see legend to fig 1.) Modified from (16) and used with permission
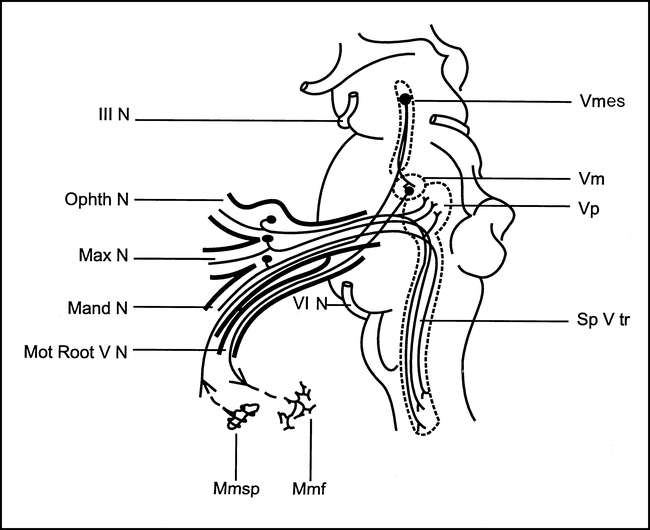
To elicit the jaw-jerk reflex, the examiner holds one finger on the subject's chin and taps it with a reflex hammer. EMG responses are recorded simultaneously from the two sides by surface electrodes placed on the masseter muscle belly (5). The jaw-jerk reflex circuit involves the ipsilateral midbrain and midpons. The afferent nerve fibers have their cell bodies in the trigeminal mesencephalic nucleus, which has collateral links with the trigeminal motor nucleus in the pons (5, 18, 19) (Fig 3).
Jaw-jerk reflex. Diagram shows the reflex arc subserving the jaw-jerk reflex. (Vmes indicates mesencephalic nucleus of the trigeminal nerve; Vp, principal sensory nucleus of the trigeminal nerve; Vm, trigeminal motor nucleus; Sp V tr, spinal trigeminal tract; III N, oculomotor nerve; Ohpth N, ophthalmic trigeminal root; Max N, maxillary trigeminal root; Mand N, mandibular trigeminal root; Mot Root V N, trigeminal motor root; VI N, abducens nerve, Mmsp, masseter muscle spindle; Mmf, masseter muscle fibers.) Modified from (5) and used with permission
Topodiagnostic implications of trigeminal reflex abnormalities have been described previously (5, 12, 15, 20).
EMG studies were reviewed by a neurologist and a neurology resident with expertise in the brain stem reflexes who had knowledge of the clinical or MR imaging findings described herein. The EMG study was considered positive when a delay in the peak or an absent latency was found. Interobserver variability was assessed with the κ statistic. Discrepancies were resolved by consensus.
MR Imaging
MR imaging was performed on a 1.5-T unit. The patients underwent a standard trigeminal nerve MR imaging protocol, which included axial proton density–and T2-weighted spin-echo images (3800/22–90/1 [TR/TE/excitations]) or fast spin-echo images (3500/22–90/1) with a 5-mm section thickness, a 23-cm field of view, and a 192 × 256 matrix of the whole brain; axial T1-weighted spin-echo images (570–610/14–15/2) with a 3-mm section thickness, a 23-cm field of view, and a 192 × 256 matrix through the pons (including the orbit and maxillary sinus), extending to the inferior mandible if the third division of the trigeminal nerve (V3) was involved; and coronal T1-weighted spin-echo images (570–610/14–15/2) with a 3-mm section thickness, a 23-cm field of view, and a 192 × 256 matrix from the midpons to the orbit apex. The T1-weighted spin-echo sequences were repeated after the intravenous injection of 0.1 mmol gadolinium per kilogram of body weight. Additional coronal and sagittal proton density–and T2-weighted spin-echo images and sagittal contrast-enhanced T1-weighted spin-echo images with the same imaging parameters as above were obtained in some patients. An MR imaging study was considered positive when a lesion was identified along the course of the symptomatic trigeminal nerve.
The MR imaging studies were independently reviewed by two neuroradiologists who were unaware of the clinical or EMG findings. Interobserver variability was assessed with the κ statistic. Discrepancies between the two observers were resolved by means of consensus.
EMG/MR Imaging Correlation
Lesion localization as demonstrated with EMG was compared with localization at MR imaging. MR imaging was the standard of reference. EMG findings were correlated with MR imaging results in a 2 × 2 table to determine the sensitivity, specificity, negative and positive predictive value, and accuracy of the EMG relative to MR imaging.
Results
The clinical, EMG, and MR imaging findings are summarized in Table 1.
Clinical, EMG reflex, and MR imaging findings in 20 patients with symptoms related to the trigeminal nerve
EMG Findings
Overall, EMG findings were abnormal in 14 trigeminal nerves (in 14 patients, unilaterally) of the 40 nerves (in 20 patients, bilaterally) examined. All abnormalities were found along the course of symptomatic nerves.
Blink reflex recordings were obtained in all 40 nerves (20 patients, bilaterally) and were abnormal in 11 symptomatic nerves (11 patients, unilaterally) and normal in nine symptomatic nerves (nine patients, unilaterally); they were also normal in all 20 asymptomatic nerves (20 patients, unilaterally).
Masseter inhibitory reflex recordings were obtained in 24 nerves (12 patients, bilaterally) and were abnormal in seven nerves (seven patients, unilaterally) and normal in five symptomatic nerves (five patients, unilaterally); they were also normal in all 12 asymptomatic nerves (12 patients, unilaterally).
Jaw-jerk reflex recordings were obtained in 22 nerves (11 patients, bilaterally) and were abnormal in four nerves (four patients, unilaterally) and normal in seven symptomatic nerves (seven patients, unilaterally); they were also normal in all 11 asymptomatic nerves (11 patients, unilaterally).
MR Imaging Findings
Eight of the 20 patients with unilateral symptoms related to the trigeminal nerve had an abnormality along the course of the symptomatic trigeminal nerve.
Five brain stem lesions were found, including infarcts in the dorsolateral medulla oblongata in three patients (in the midpons in or near the principal trigeminal nucleus in one patient) and hemorrhage in the midpons, which included the principal nucleus in another patient. Three extraaxial lesions were found, including inflammation of the infraorbital nerve in the first patient, inflammation of the trigeminal ganglion extending into the proximal mandibular division in the second patient, and a cerebellopontine angle cistern epidermoid in the third patient.
No abnormalities were seen along 12 symptomatic nerves (12 patients, unilaterally) or in any of the 20 asymptomatic nerves (20 patients, unilaterally). Six of the 12 patients with negative MR imaging studies had similar clinical findings after 3 to 36 months (mean, 14 months), and one of these six had a repeat MR imaging examination after 36 months, which was also negative. Four were lost to follow-up, and two had no symptoms, one after 4 months and one after microvascular decompression for trigeminal neuralgia.
EMG/MR Imaging Correlation
The results of the EMG/MR imaging correlation of the 40 trigeminal nerves examined are summarized in Table 2.
EMG versus MR imaging results in 40 trigeminal nerves (20 symptomatic)
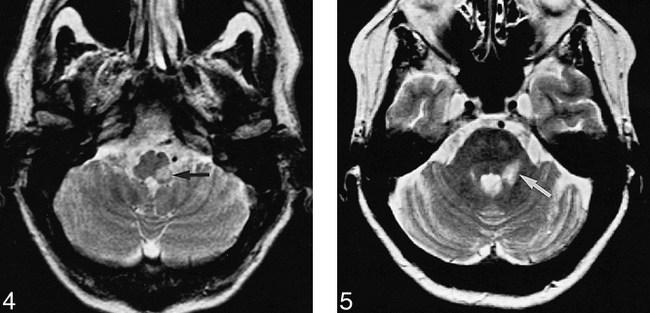
There were 14 positive EMG studies, corresponding to eight positive and six negative MR imaging studies. Of the eight patients with positive EMG and MR imaging studies, five had brain stem lesions. In these five patients, lesion localization, as demonstrated with EMG, corresponded well with lesion localization as shown by MR imaging (Figs 4 and 5). Three of these eight patients had extraaxial lesions; EMG correctly identified these lesions, but could not exactly localize the abnormalities as MR imaging did (Fig 6). In six patients, EMG was positive and MR imaging was negative. At clinical follow-up, three of these six patients had similar symptoms after 5, 12, and 36 months, respectively. One had a repeat MR imaging study, which was also negative; the other three were lost to follow-up. None of the trigeminal nerves that produced a negative EMG study showed a positive MR imaging result. As compared with MR imaging, EMG had a sensitivity of 100% (95% confidence interval [CI]: 63% to 100%), a specificity of 81% (95% CI: 64% to 93%), a positive predictive value of 57% (95% CI: 29% to 82%), and a negative predictive value of 100% (95% CI: 87% to 100%) (Table 2).
Patient 2: 52-year-old man with a sensory deficit in the first, second, and third divisions of the left trigeminal nerve and a sensory deficit on the right side of the body. Blink reflex recorded to left supraorbital nerve stimulation evoked a normal R1 response, whereas R2 responses were bilaterally delayed. R1 and R2 reflex recordings to right supraorbital nerve stimulation were normal. This lesion corresponds to the location of the left descending trigeminal tract and its nucleus in the dorsolateral part of the medulla oblongata. Axial T2-weighted MR image confirms the presence and location of the lesion in the left dorsolateral part of the medulla oblongata (arrow).
fig 5. Patient 3: 56-year-old woman with a sensory deficit in the second division of the trigeminal nerve. Blink reflex recorded to left supraorbital nerve stimulation showed the R1 to be absent and the R2 components delayed bilaterally. This lesion corresponds to the location of the left principal sensory nucleus in the pons with involvement of the descending trigeminal tract. Axial T2-weighted MR image (3500/90) confirms the location of the lesion in the left lower dorsal half of the pons, including the region of the left principal sensory nucleus (arrow).
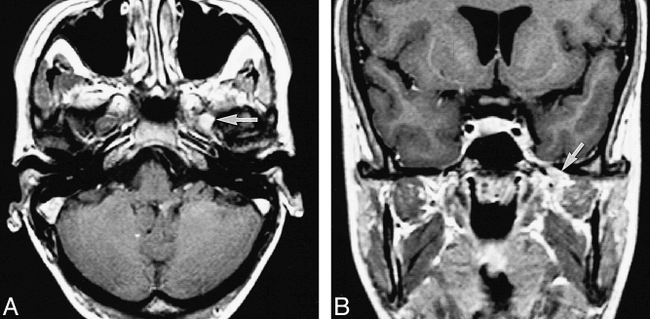
Patient 13: 25-year-old woman with pain in the first trigeminal division. Sensory loss was found in all three trigeminal divisions, and oculomotor, abducens, and facial nerve palsies were present. Blink reflex recorded to left supraorbital nerve stimulation showed a delayed R1 and normal R2 components. The left jaw-jerk reflex response was absent. The left masseter inhibitory reflex showed an afferent delay (ie, a bilateral delay of the first and second silent periods). All reflex responses were normal after stimulation of the right side. The abnormal reflex findings corresponded to a proximal trigeminal nerve lesion, probably at the root level.
A and B, Axial (A) and coronal (B) contrast-enhanced T1-weighted MR images show enhancement and thickening of the trigeminal ganglion and proximal third division of the trigeminal nerve in the foramen ovale (arrows). Follow-up MR imaging studies (not shown) and clinical findings showed resolution of the abnormalities, indicating an inflammatory lesion.
There was a high degree of agreement between the observers with regard to the presence of an appropriate lesion at EMG (κ = .94) and at MR imaging (κ = .92).
Discussion
Previous authors have shown that clinical localization of a trigeminal nerve lesion is poor as compared with MR imaging results and that the entire course of the trigeminal nerve should always be visualized (4). The results of our study indicate that electrodiagnostic testing of the trigeminal reflexes can be used for improved lesion localization. EMG of multiple trigeminal nerve reflexes can accurately localize lesions in the brain stem because the trigeminal reflex circuits are located at different brain stem levels–-mesencephalon (jaw-jerk reflex); pons (blink reflex-R1, masseter inhibitory reflex-SP1); pontomedullary junction (masseter inhibitory reflex-SP2); and lower medulla (blink reflex-R2)–-enabling accurate assessment of these regions (5, 11, 12, 15, 16, 18, 20).
In six of our patients, EMG reflex studies were positive and MR imaging studies were negative. Very small lesions or impaired physiologic functions may be found with trigeminal reflex testing, even though structural abnormalities cannot be depicted with MR imaging (20, 21). This may explain the relatively high number of positive (6/14) EMG findings relative to MR imaging results in our study. We postulate that in some of these patients the lesions were too small to be seen with MR imaging. Probable causes for the trigeminal nerve deficits in these patients are microinfarctions in the brain stem, ischemia of the nerve itself, viral inflammation, and autoimmune disorders (10, 22, 23).
Previous investigators have noted that EMG can distinguish between idiopathic trigeminal neuralgia (ie, neurovascular) and symptomatic trigeminal neuralgia (ie, due to a structural cause, such as neoplasms or multiple sclerosis) (8, 24). Cruccu et al (24) recorded the trigeminal reflexes, including blink, masseter inhibitory, and jaw-jerk, in 30 patients with idiopathic trigeminal neuralgia and in 20 patients with symptomatic trigeminal pain. Of the 30 patients with idiopathic trigeminal neuralgia, only two showed slight delays of short-latency reflexes. In the other 28 cases, the trigeminal reflexes were completely normal. These results correspond well with the negative EMG studies in the patient with trigeminal neuralgia in our study. In the study by Cruccu and coworkers, all the patients with symptomatic trigeminal pain had trigeminal reflex abnormalities (24).
The results of our study indicate that EMG reflex studies can play a role in the preselection of patients with trigeminal nerve dysfunction who are referred for MR imaging. The high negative predictive value of EMG suggests that if a normal EMG result is obtained, it is unlikely that MR imaging will show a structural cause for the symptoms and is therefore not required for this purpose. Nonetheless, the high sensitivity and negative predictive value of EMG in predicting MR imaging results in our study may be due to selection bias of the patients in this retrospective study. The prevalence of positive MR imaging studies was relatively low in our series (abnormalities found in 40% of all patients and in 20% of all nerves examined) as compared with a previous study of 112 consecutive patients who underwent MR imaging for symptoms and signs related to the trigeminal nerve (6). In that study, abnormalities along the trigeminal nerve were found in 68 patients (61%) (6). Furthermore, to compare EMG reflex and MR imaging studies of the trigeminal nerve in a blinded manner, the trigeminal nerves of the clinically normal sides were also included in our study, which contributed to the low prevalence.
To our knowledge, assessment of interobserver variability of EMG reflex studies of the trigeminal nerve has not been performed previously. The good interobserver agreement for MR imaging we obtained corresponds to the results of a previous study (6). The high κ values for both the EMG reflex studies and the MR imaging examinations indicate that these are both robust tests for evaluating trigeminal nerve lesions.
Conclusion
Our data suggest that EMG of the trigeminal reflexes can be used to exclude structural lesions and to localize accurately lesions of the trigeminal system in the brain stem. When a lesion is localized in the brain stem with EMG reflex studies, a more tailored MR examination of this region might be sufficient. We recognize, however, that our study represents preliminary work and that a large prospective study is warranted to validate these conclusions.
Footnotes
↵1 Address reprint requests to Charles B.L.M. Majoie, MD, PhD, Department of Radiology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
References
- Received June 19, 1998.
- Accepted after revision January 15, 1999.
- Copyright © American Society of Neuroradiology