Abstract
Summary: We report the MR findings in two patients with clinically and histologically proved Dejerine-Sottas disease. One patient had spinal involvement with multiple thickened and clumped nerve roots of the cauda equina; the second had multiple enlarged and enhancing cranial nerves. Although these findings are not specific for Dejerine-Sottas disease, they are suggestive of the diagnosis, which is further corroborated with history and confirmed with sural nerve biopsy and laboratory studies.
Dejerine-Sottas disease is a rare hereditary motor and sensory neuropathy (HMSN type III) that presents with distal extremity motor and sensory symptoms as well as palpable peripheral nerves. Cranial nerve involvement is reportedly seen in approximately 15% of cases (1). MR imaging is considered the technique of choice for determining the extent of CNS involvement. Because of its rarity, there is a paucity of literature regarding the imaging characteristics of this disorder. We report two patients with histologically proved diagnoses of HMSN type III and describe the MR imaging findings, which may suggest the diagnosis.
Case Reports
Case 1
At 4 months of age, an infant boy was noted to have atrophy of the muscles of his lower extremities, with poor muscle tone and absent deep tendon reflexes. Subsequent motor development milestones were delayed, despite advanced social and language skills. At age 2 years, the patient underwent electromyography, nerve conduction studies, and a quadriceps muscle biopsy. These studies revealed denervation atrophy without intrinsic muscle abnormality. A sural nerve biopsy was also performed, which revealed hypertrophic “onion bulb” neuropathy with virtual lack of myelin, compatible with the Dejerine-Sottas type hereditary sensory and motor neuropathy. At 7 years of age, the patient underwent an MR examination of the lumbar spine (Fig 1) for progressive lower extremity weakness.
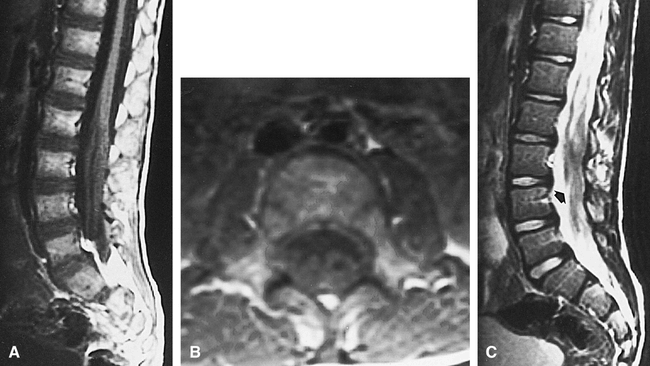
Case 1: 7-year-old boy with spinal nerve enlargement.
A–C, Sagittal T1-weighted (600/11/2 [TR/TE/excitations]) (A), axial T1-weighted (600/11/2) (B), and sagittal T2-weighted (2500/96/2) (C) images show marked enlargement of the nerves of the cauda equina (arrow, C).
The MR study revealed markedly abnormal thickening and clumping of the spinal nerve roots of the cauda equina (Fig 1); however, signal intensity of the enlarged nerve roots was normal. The conus terminated somewhat low, at the level of L3. The cervical and thoracic portions of the cord were unremarkable. Contrast material was not administered.
After the diagnosis, the patient was entered into an intensive physical therapy and rehabilitation program. On recent follow-up, at 10 years of age, he was dependent on a walker or modified crutches for ambulation. He remains ataxic and has substantial weakness in both upper and lower extremities. He continues to undergo intensive physical therapy.
Case 2
At 1 year of age, an infant boy was found to have delayed motor development milestones, and was slow to walk. He underwent muscle and nerve biopsies as a young child, which revealed hypertrophic demyelinating neuropathy, compatible with Dejerine-Sottas disease. He had intensive physical therapy, as well as bilateral ankle fusions to treat severe footdrop, but eventually became wheelchair-bound by the time he entered college. He came to our institution at age 23 years with severe eye pain, and was found to have mild bilateral facial weakness, greater on the left side. There was no ocular motility imbalance or facial numbness. He had significant atrophy of all muscle groups of his extremities, and deep tendon reflexes were absent. He underwent a contrast-enhanced MR examination of the head (Fig 2).
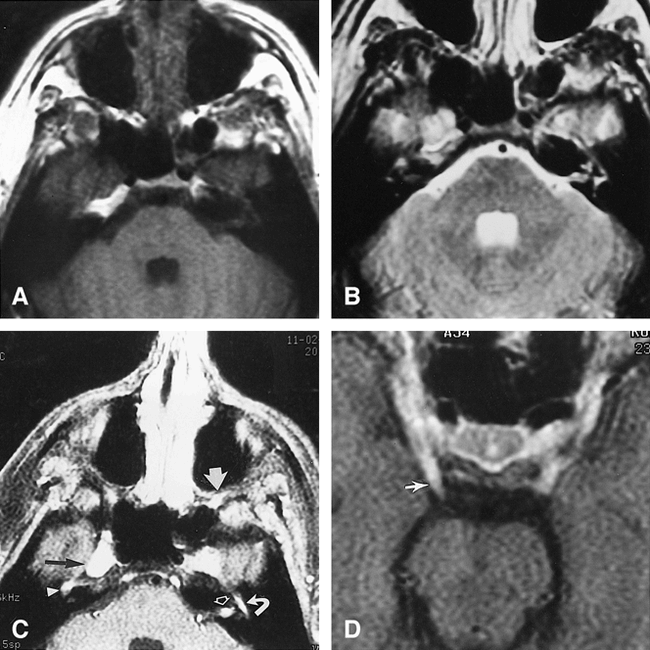
Case 2: 23-year-old man with enlarged cranial nerves
A–D, Axial noncontrast T1-weighted (600/15/1) (A) and axial spin-echo T2-weighted (2500/96/1) (B) images are not as revealing as the axial contrast-enhanced T1-weighted (600/15/1) images (C and D). There is prominent enhancement in the left porous acousticus (open arrow, C), enlargement and enhancement of the fifth cranial nerves in Meckel's cave, slightly more prominent on the right (black arrow, C) than left, and an enlarged enhancing branch of the maxillary nerve in the pterygopalatine fossa on the left (wide white arrow, C). The right geniculate ganglion (arrowhead, C) and the tympanic portion of the left seventh nerve (curved arrow, C) are enlarged and enhancing. Also evident are bilateral enlargement and enhancement of the third nerve, more prominent on the right side (arrow, D).
The MR study revealed abnormal prominence of the fifth cranial nerves in the region of Meckel's cave (Fig 2). The third, fifth, and seventh cranial nerves all exhibited enlargement and abnormal enhancement bilaterally. The remainder of the brain was unremarkable.
The patient's eye pain was thought to be attributable to a corneal abrasion, which was unrelated to his underlying condition. His symptoms resolved with topical medications and he has not returned for follow-up imaging.
Discussion
Hypertrophic neuropathy may occur in a number of diseases, including neurofibromatosis, Charcot-Marie-Tooth disease (HMSN type I), diabetes mellitus, acromegaly, amyloidosis, leprosy, Guillain-Barré syndrome, and Dejerine-Sottas disease (HMSN type III). Progressive hypertrophic interstitial neuropathy of childhood, also known as Dejerine-Sottas disease or onion bulb neuropathy, was first described in 1893 (2, 3). Dejerine-Sottas is a rare autosomal recessive condition, with occasional sporadic cases, that encompasses a particular constellation of unique clinical, laboratory, and histologic findings. The disease is characterized by an early-onset demyelinating neuropathy, and usually manifests as gradual progression of distal weakness, sensory loss, and areflexia in the legs. As the disorder progresses, involvement extends to the upper extremities, and palpable peripheral nerves become evident. Other reported symptoms include cranial nerve deficits, tremors, and general ataxia. Occasionally, spinal cord compression results from hypertrophic neuropathy of the spinal roots (4).
Nerve conduction studies reveal severe slowing of peripheral nerve conduction, with denervation changes in the associated muscle groups. CSF analysis has frequently shown elevated protein concentration. Sural nerve biopsy specimens show hypomyelination, classic onion bulbs composed of whorls of Schwann cell processes that are characteristic of Dejerine-Sottas disease (5) but that are occasionally seen in HMSN type I. Histologic specimens from Dejerine-Sottas patients, however, consistently show greater frequency of onion bulb changes and a lower density of myelinated fibers than seen in patients with HMSN type I (Charcot-Marie-Tooth disease). Patients with Dejerine-Sottas disease also have a much higher prevalence of clinically enlarged peripheral nerve roots than those with HMSN type I or type II, which are both variants of Charcot-Marie-Tooth disease. Type II Charcot-Marie-Tooth syndrome presents later in life than type I HMSN and the disease is usually less severe with a lesser degree of neuronal enlargement. The Table compares and contrasts HMSN types I, II, and III.
Comparison of HMSN types I, II, and III
Radiographically, the disease can appear as scalloping of the vertebral bodies with triangulation of pedicles and enlarged intervertebral foramina (6). Myelographically, the disease may be suggested by grossly enlarged spinal nerve roots and occasionally by myelographic block (7). Less common findings include oblique tubular and rounded intradural/extramedullary filling defects in the contrast column due to the enlarged bulbous nerve roots (4, 7).
Two previous case reports have described the MR imaging appearance of this disease, although both depicted only involvement of peripheral nerves (8, 9). Both cases reported grossly hypertrophied peripheral nerves/roots, which demonstrated abnormal foci of high T2 signal intensity, suggesting edema and/or demyelination. Neither study used contrast material.
Conclusion
The findings of thickened nerve roots of the cauda equina and enlarged enhancing cranial nerves are not pathognomonic for Dejerine-Sottas disease, and may be observed in a number of congenital disorders, such as neurofibromatosis (crossing over into neoplastic disorders) and HMSN type I, and infectious/inflammatory causes, including Guillain-Barré syndrome, sarcoidosis, arachnoiditis, and meningitis. Of the neoplastic disorders, subarachnoid seeding, multiple schwannomas, and lymphomatous/leukemic infiltration may be entertained in the differential diagnosis. Acquired illnesses, such as acromegaly or amyloidosis, may be associated with enlarged nerve roots. Although our findings are not specific for Dejerine-Sottas disease, they may be highly suggestive in young children with congenital neuropathies. The clinical presentation, subsequent CSF and nerve conduction studies, and sural nerve biopsies should corroborate the diagnosis of Dejerine-Sottas disease.
Footnotes
↵1 Address reprint requests to David M. Yousem, MD, Department of Radiology, Johns Hopkins Hospital, Houck B-112, 600 N Wolfe St, Baltimore, MD 21287.
References
- Received July 1, 1998.
- Copyright © American Society of Neuroradiology