Abstract
BACKGROUND AND PURPOSE: Brain temperature may be an important factor governing the extent of neuronal injury associated with stroke. The goal of this study was to develop a noninvasive method for measuring brain temperature, both to characterize the extent to which temperature changes after stroke and to test protocols designed to reduce brain temperature. We used an animal model to test the ability of 1H MR spectroscopy to measure temperature from infarcted brain tissue at 24 hours after insult.
METHODS: Unilateral permanent focal ischemia in the middle cerebral artery territory was induced in adult dogs by intravascular delivery of microfibrillar collagen. MR imaging performed at 24 hours after insult was used to guide the implantation of temperature probes into the basal ganglia infarct and into the same anatomic location on the contralateral side. Serial non-water-suppressed 1H MR spectra were obtained from 1.3-cm3 voxels using an echo time of 136 and 272 ms, alternately, from the infarcted and contralateral non-infarcted tissue during a period when brain temperature was raised and lowered by whole-body heating and cooling.
RESULTS: The chemical shift difference between the 1H MR spectroscopy signal of water and N-acetylaspartate or water and trimethylamines was plotted against brain temperature for two voxel locations. The slope and intercept of the plots obtained for infarcted and non-infarcted brain were not significantly different (P < .05, t test), and there was no difference between the slope and intercept of plots made from data collected with an echo time of 136 or 272 ms.
CONCLUSION: The results of this study indicate that brain temperature can be measured from regions of brain containing infarcted tissue, at least up to 24 hours after ischemia. It should be possible to apply the 1H MR spectroscopy method used in the present study to measure brain temperature after stroke.
During the first week after stroke, there is a correlation between increased body temperature and decreased survival or neurologic recovery (1–3). Studies using animal models have shown that elevations in brain temperature of 1° to 2° C worsen neurologic and histologic indicators of ischemic brain damage (4, 5), and similar magnitude decreases during or after the insult diminish damage (6–8). Several different types of protocols have been investigated in animal models to induce hypothermia, including whole-body cooling (6–8), localized head cooling (9, 10) and drug-induced cooling (11, 12). Positive results from these studies have provided the justification for clinical trials of whole-body cooling as a neuroprotective strategy after head injury (13) and raise the suggestion that similar approaches could be applied to reduce brain injury in patients after stroke (14), cardiac arrest (15), or neonatal hypoxic ischemia (16). It remains unclear, however, whether cooling protocols developed for and applied to animal models would be effective for reducing brain temperature in humans. The opportunity to measure brain temperature in humans directly has been limited to rare situations involving ventriculostomy, in which a temperature probe was implanted alongside each catheter (17–19). In such situations, the anatomic location of the temperature measurement site has been limited to the site where each catheter was implanted. It has not been possible to measure brain temperatures in different locations, such as the primary site of injury, sites on the perimeter of the injury, or uninjured tissue.
We have shown that brain temperature can be measured using 1H MR spectroscopy to quantify the MR frequency of water, which changes as a linear function of temperature (20–22). An attractive feature of this method is that temperature from virtually any region of the brain can be measured, allowing the possibility of examining the effectiveness of different protocols to reduce brain temperature in a variety of anatomic locations. A second feature is that the procedure is noninvasive, allowing direct brain temperature measurements in humans. A third feature the relationship between temperature and the chemical-shift difference of the MR signals corresponding to H2O and a reference signal, such as N-acetylaspartate (NAA) and related compounds or trimethylamines (TMA), remain constant before, during, and after cerebral ischemia, as measured up to 90 minutes after ischemia. The possibility remains, however, that this relationship could change during a longer interval after stroke. There could be changes in the magnetic susceptibility experienced by H2O relative to NAA and TMA within a voxel, with a consequent change in the relationship between the water and reference peaks. Ischemia may induce alterations in the concentrations of the chemical species, giving rise to the NAA and TMA MR peaks, resulting in a change in the chemical shift of the in vivo MR peak. Confirming that accurate temperature readings can be obtained from ischemically injured brain tissue after a longer post-insult period is important because stroke patients may not be sufficiently stable to allow transport to an MR facility until several hours after the initial insult.
The primary goal of this study was to test the hypothesis that the relationship between brain temperature and the chemical shift-difference between the MR spectroscopy peaks, which correspond to H2O and NAA or H2O and TMA, differs in normal non-infarcted brain versus infarcted brain tissue when measured at 24 hours after insult. A secondary goal was to assess these same relationships using an echo time (TE) of 272 ms for MR spectroscopy data collection, because this TE is commonly used for the collection of 1H MR spectroscopy data from humans (23–26).
Methods
Surgical Preparation and Experimental Protocol
Surgical procedures and the experimental protocol were approved by the University of Texas Southwestern Medical Center Institutional Review Board for Animal Research and were in accordance with the National Institutes for Health Guide for the Care and Use of Laboratory Animals. The model of canine cerebral infarction has been described in detail previously (27, 28) and was followed with minor modifications as detailed below. On the day before the MR studies were initiated, adult mongrel dogs (n = 5) were anesthetized (thiamylal sodium, 20 mg/kg of body weight, IV administration), intubated, and ventilated with 2% to 5% isoflurane (balance air). The right femoral artery in the groin was surgically exposed, and the tip of a transfemoral catheter was positioned 1 to 2 cm proximal to the bifurcation of the left common carotid artery, under fluoroscopic observation. To induce middle cerebral artery (MCA) occlusion, 30 to 50 mg of microfibrillar collagen (Avitene; Alcon Laboratories, Ft. Worth, TX) suspended in 0.5 mL of normal saline was introduced into the catheter and flushed with saline. The catheter was removed, the surgical site sutured, and the ventilation mixture switched to 100% air. The animal was allowed to awaken, was extubated, and was returned to a holding room until the next day. Analgesia (0.02 mg/kg buprenorphine, intramuscular administration) and penicillin (600,000 units, intramuscular administration) were administered to the animal, and the animal was transported to a holding area and observed periodically during the ensuing ∼18 hours. The next morning, the animal was transported to the MR facility and a neurologic evaluation was performed using a standardized categorical rating scale, described in detail previously (29), to evaluate motor function (no deficit = 1, hemiparetic but able to walk = 2, stands only with assistance = 3, hemiplegia and unable to stand = 4), consciousness (normal = 1, mildly reduced = 2, severely reduced = 3, comatose = 4), head turning (absent = 0, posturing and turns toward side of infarct = 1, comatose = 1), circling (absent = 0, present = 1, does not walk = 1), and hemianopsia (absent = 0, present = 1, unable to test because of reduced consciousness = 1). According to this scale, a normal animal would have a composite score of 2 and an animal with the most severe deficits would have a score of 11. Next, the animal was anesthetized, intubated, and ventilated as described above. A second dose of buprenorphine was administered, and pancuronium bromide (0.3 mg/kg per hour, IV administration) was given for muscle relaxation to ensure no movement during MR measurements. A femoral arterial catheter was used to monitor heart rate and blood pressure and to draw blood for PCO2, PO2, and pH. The scalp was retracted, and two burr holes were made in the skull for subsequent implantation of temperature probes into the infarcted and contralateral hemispheres.
On the morning of the study, three fiber optic temperature sensors (model MIA sensors and a model 750 monitor; Luxtron, Mountain View, CA) were calibrated at room temperature, relative to a type T copper/constantan thermocouple and thermometer (Cole-Palmer, Chicago, IL). At the end of each study, the calibration of the three sensors was retested; the average change in the readings compared with the initial calibration was 0.04 ± 0.20°C. After transporting the animal to the magnet, it was wrapped in a thermal blanket with thermostatically controlled circulating water passing through it, and one temperature sensor was inserted to measure rectal temperature. The MR coil was placed around the head, and the animal was placed in the magnet for MR imaging to identify infarcted regions of brain for subsequent 1H MR spectroscopy measurements. The animal was then removed from the magnet, and with the MR coil left in place, two temperature sensors were implanted 1.5 to 2.5 cm into the brain. The exact depth and location of probe implantation was guided by the MR images, with one sensor implanted into the region of infarct and the other sensor implanted contralateral to the infarct in the analogous anatomic location. The animal was then returned to the magnet and reimaged, and voxels were selected for MR spectroscopy measurements. During the MR imaging and temperature sensor implantation, the rectal temperature was maintained at 39°C, and the ventilation rate and tidal volume were adjusted to maintain arterial blood PO2 and PCO2 readings in the range of 100 to 200 and 30 to 45 mmHg, respectively. During the next 3 to 4 hours, the temperature of the animal was gradually increased or decreased by passing warm or cool water through the blanket surrounding the animal, with concurrent recordings of rectal and brain temperature from the sensors and 1H MR spectroscopy data collection from the infarcted and non-infarcted contralateral brain regions. Arterial blood pH, PO2, and PCO2 were measured every 60 minutes, and heart rate and mean arterial blood pressure were recorded every 30 minutes. At the end of the MR study, the animal was euthanized by pentobarbital overdose (120 mg pentobarbital/kg, IV administration). To confirm the location of the infarct measured by MR imaging and MR spectroscopy, the brain was removed and 5-mm-thick sections obtained from the coronal plane were stained with 2,3,5-triphenyl, 2-H tetrazolium chloride (TTC) as described previously (30). Previous studies have shown the concordance between infarct locations identified by the absence of TTC staining and conventional histologic staining methods for this and similar models (31, 32).
MR Data Collection and Data Analysis
All MR experiments were performed using a 1.5-T Philips NT system (Philips Medical Systems, Shelton, CT) and the manufacturer's knee imaging coil as described previously (22). Imaging consisted of a modified rapid acquisition with relaxation enhancement, turbo spin-echo pulse sequence to provide T2-weighted images (2.0 × 0.6 × 0.7-mm resolution) in the sagittal and coronal (perpendicular to the corpus callosum) planes (10 and 20 sections, respectively). These images were used to guide the selection of two 1.3-cm3 voxels for 1H MR spectroscopy measurements from the infarcted and non-infarcted cerebral hemispheres, respectively. Non-H2O-suppressed 1H MR spectra were obtained using a point-resolved spectroscopy sequence with a sweep width of 750 Hz and 1024 data points per spectrum. The delay time between excitation pulses was 2.7 seconds, and the TE was 136 or 272 ms. After four dummy images were obtained to establish partial saturation, 64 or 128 free induction decays were accumulated for each spectrum in 3 or 6 minutes, respectively. The accumulated 1H free induction decays were processed by baseline correction to remove direct current offsets, 2 Hz exponential apodization, zero filling to 16,384 data points (final digital resolution = 0.0007 ppm/data point), Fourier transformation, and manual zero-order phasing to provide the maximum positive intensity for both the H2O and NAA nuclear MR peaks. The chemical-shift scales of all spectra were calibrated with respect to the location of the NAA 1H MR peak set to 2.02 ppm. The exact chemical shifts corresponding to the H2O, TMA, and NAA MR peaks were determined using the “single peak analysis” software that is routinely supplied with the spectrometry system (versions 5.1 and 5.2 operating systems). Using linear regression analysis, a total of 67 MR spectra from the five dogs were collected to investigate the relationship between chemical shift and brain temperature: 35 spectra (contralateral, n = 15; infarcted, n = 20) using a TE of 136 ms, and 32 spectra (contralateral, n = 16; infarcted, n = 20) using a TE of 272 ms. For data collected at either TE, brain temperature measured from the implanted probes was plotted versus the chemical-shift (δ) difference between the 1H nuclear MR frequencies of water and NAA (Δ δH2O − δNAA) and between water and TMA (Δ δH2O − δTMA), and statistical tests were performed to test for different slopes and elevations (33). All data are presented as a mean ± SD for the five animals studied unless otherwise noted.
Results
The neurologic assessment made at 20 to 22 hours after the MCA embolization revealed abnormalities in four of the five animals, with the composite neurologic scores ranging from 6 to 10. In the four abnormal animals, there were deficits in motor function (score range, 2−4), consciousness (score range, 2−3), head turning (score, 1), and circling (score, 1), and in three animals, hemianopsia (score, 1) was present. The neurologic status of a fifth animal was normal, and subsequent MR imaging measurements, TTC staining, and examination of the brain of this animal revealed no evidence of infarction. As observed previously, in approximately 10% of animals, the injected collagen apparently occludes the external carotid artery rather than the MCA territory and does not result in ischemic injury (29). For this fifth animal, a single temperature probe was implanted into the same vicinity of brain as for other animals, and the resulting data collected during the protocol were combined with the data collected from the contralateral regions for the other four animals.
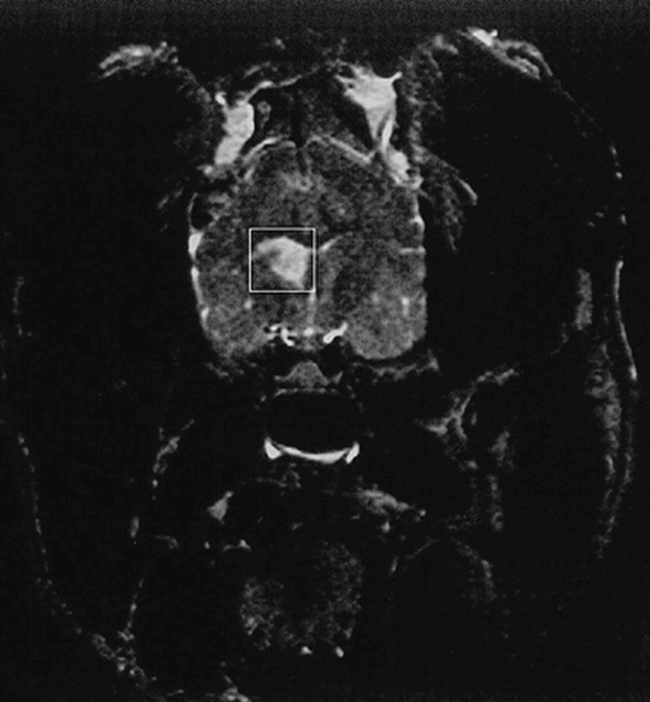
Arterial blood pH (7.21 ± 0.04), PO2 (163 ± 29 mmHg), PCO2 (33 ± 3 mmHg), heart rate (134 ± 20 bpm), and mean arterial blood pressure (120 ± 18 mmHg), recorded at the beginning of MR data collection, indicated that all of the animals were physiologically stable. These readings remained constant throughout the protocol. The MR images obtained from the four animals with neurologic deficits revealed abnormalities in the cerebral hemisphere ipsilateral to the collagen injection (Fig 1). In all four animals, T2-weighted MR images revealed the presence of unilateral hyperintense signal in the MCA territory, comprising the basal ganglia of one hemisphere. In one animal, there was also extensive hyperintensity present in the cerebral cortex of the same hemisphere (not shown). Subsequent removal and TTC staining of the brains of these animals at the end of the protocol revealed decreased uptake of TTC in the same regions showing hyperintense signal in the MR images and confirmed the presence of unilateral nonhemorrhagic infarcts.
1H MR image of canine brain. A T2-weighted coronal image, obtained 24 hours after MCA embolization, shows hyperintense signal from the basal ganglia. The box shows the location of the voxel used for 1H MR spectroscopy data collection from the infarct; the same-sized voxel was collected from the analogous anatomic region contralateral to the infarct.
The average temperatures recorded from contralateral brain tissue and rectum at the start of 1H MR spectroscopy data collection were 39.1 ± 1.1 and 38.9 ± 1.2°C, respectively. Temperatures recorded throughout the protocol indicated a systematic difference between the cerebral temperatures recorded from the two implanted temperature probes for three of the four animals with infarcts. In one animal, the average difference (ie, infarct minus contralateral) was 0.07 ± 0.09°C (paired t test, P = .01), whereas in two animals, the average difference was −0.39 ± 0.15 and −0.40 ± 0.19°C (P < .0005).
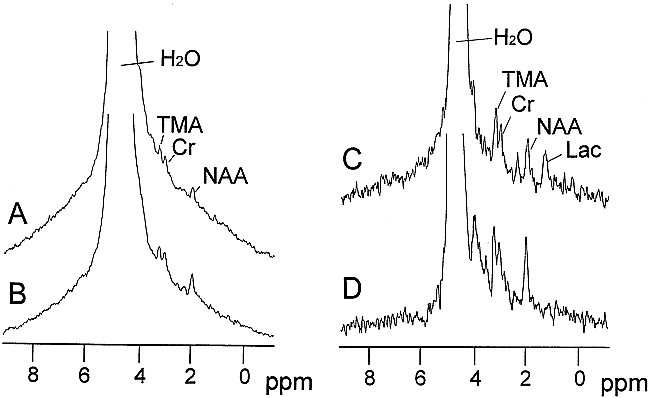
Non-water-suppressed 1H MR spectroscopy data were collected from a voxel centered on the infarct located in the basal ganglia and from the same-sized volume contralateral to the infarcted hemisphere (Fig 2). MR spectroscopy peaks corresponding to H2O, TMA, creatine (Cr) plus phosphocreatine, and NAA were visible in both the infarcted and non-infarcted tissue, although the signal from NAA relative to creatine and TMA appeared to be approximately 20% to 50% lower in the former compared with the latter tissue. A peak located at 1.3 ppm, assigned to lactate, was also present in MR spectroscopy spectra collected using a TE of 272 from the infarct (Fig 2C).
Representative 1H MR spectra from infarcted and contralateral non-infarcted brain tissue using TEs of 136 ms and 272 ms (data from the same animal as that depicted in fig 1). The MR signals corresponding to H2O, TMA, creatine plus phosphocreatine, NAA, and related compounds, and lactate are identified. A five-times larger vertical scaling factor was used for the data collected using a TE of 272 ms, compared with the data collected using a TE of 136 ms.
A, 1H MR spectra of infarcted brain tissue (TE, 136).
B, 1H MR spectra of non-infarcted brain tissue (TE, 136).
C, 1H MR spectra of infarcted brain tissue (TE, 272).
D, 1H MR spectra of non-infarcted brain tissue (TE, 272).
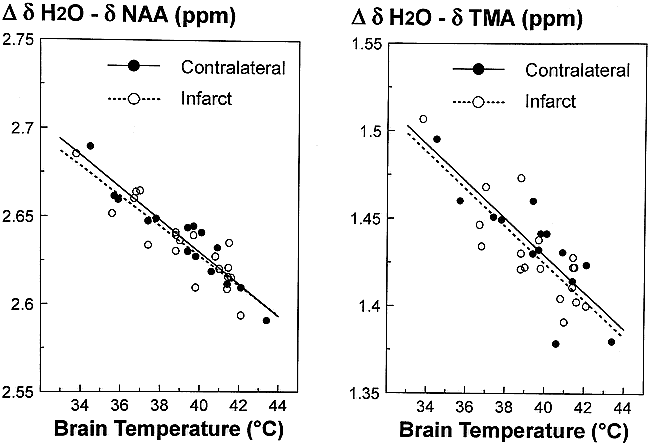
For 1H MR spectroscopy data collected using a TE of 136, there was a significant linear relationship (P < 0.05) between the chemical shift differences Δ δH2O − δNAA or Δ δH2O − δTMA versus brain temperature (Fig 3). A comparison of the best-fit linear regression lines revealed no significant difference between the slope and elevations for noninfarcted compared with infarcted tissue, for both Δ δH2O − δNAA and Δ δH2O − δTMA versus brain temperature relationships (t test, P > .05). A similar comparison using data collected with a TE of 272 ms yielded the same findings. A comparison of combined data (infarcted and noninfarcted) revealed no difference in the slope or intercept of data collected using a TE of 136 ms versus a TE of 272 ms. The best fit by linear regression for combined data (ie, TE 136 and 272 plus non-infarcted and infarcted) gives: TNAA = −82.33 ± 6.58 (Δ δH2O − δNAA) + 255.94 ± 1.30 (r2 = 0.71) and TTMA = −70.11 ± 6.06 (Δ δH2O − δTMA) + 140.03 ± 1.31 (r2 = 0.69).
Comparison of the relationship between brain temperature measured from sensors implanted into the infarcted and contralateral non-infarcted tissue versus the chemical-shift differences between H2O and NAA (left) and between H2O and TMA (right). Only data collected using a TE of 136 ms are depicted. The lines show the best fit by linear regression analysis. There was no significant difference in the slope or elevation of the data collected from the infarct compared with the non-infarcted tissue.
Discussion
The use of 1H MR spectroscopy to measure brain temperature is contingent on identifying and measuring the location of at least one of the two reference peaks, NAA or TMA. One of our early concerns was that there would be insufficient signal intensities from NAA to be used as a chemical shift reference, because NAA decreases during the first several hours after stroke (24–26, 32, 34). Although we observed lower relative NAA signal intensities for the voxel centered on the infarct, the signal from NAA was still sufficient to make peak assignments and quantify the chemical shift unambiguously. It is conceivable that the signal from NAA measured from this voxel was derived from peripheral noninfarcted tissue because of partial volume averaging. We could have mitigated partial volume averaging effects somewhat by using the MR instrument's minimum allowed voxel size (1 cm3). This, however, would have required imaging times that were approximately 4.8 times longer and would have prevented our goal of collecting MR spectroscopy data over a range of different brain temperatures, but with constant temperatures during each imaging session, within our allotted time on the MR instrument. Alternatively, attempting to create a larger volume of infarct by injecting a greater volume of collagen would substantially decrease the chances of survival for 24 hours (27, 28). The temperature-chemical-shift difference relationship examined using TMA as the chemical shift reference should not be as subject to the problem of partial volume averaging; previous studies have shown that TMA either remains constant or increases after stroke (24, 34). The fact that similar slopes and intercepts were obtained from infarcted and noninfarcted tissue when using NAA or TMA as the reference peak supports the conclusion that the relationship between temperature- and chemical-shift difference is unaffected by infarction.
It has been shown that the magnetic susceptibility effects on muscle (35) and brain (36, 37) can affect the determination of temperature using phase-contrast MR imaging, which, as does the present method, assumes linear temperature-dependent changes in the MR frequency of water. The accuracy of the present method should not be impaired by magnetic susceptibility as long as the water and reference peaks are equally effected. The concurrence of slopes and intercepts for infarcted and noninfarcted tissues obtained in the present study implies that changes in magnetic susceptibility in brain tissue after stroke, if any, were not sufficient to affect the temperature-chemical shift difference relationship. It is worth noting that the four abnormal animals studied were judged to have nonhemorrhagic embolic strokes in the MCA territory. Although not addressed herein, it is possible that a hemorrhagic stroke could generate significantly greater magnetic susceptibility effects, which could impact the ability of the MR spectroscopy method to measure temperature to a greater degree than that observed in this study. It may be possible to use our canine model to examine the ability of 1H MR spectroscopy to measure temperature in hemorrhagic infarcts, because this model gives an increasing proportion of hemorrhagic infarcts as the interval between the MCA embolization increases beyond 24 hours (27, 29).
The in vivo TMA 1H MR peak in the dog brain likely derives the bulk of its signal from glycerophosphocholine, with minor contributions from phosphocholine and choline, whereas the NAA peak derives its signal primarily from NAA, with minor contributions from N-acetylaspartylglutamate, glutamate, and acetate (38). It is conceivable that the relative contribution of these compounds to the in vivo signal could change in infarcted compared with noninfarcted tissue, causing a change in the location of the NAA and TMA reference peaks relative to water. This, in turn, would cause a systematic error in the calculation of brain temperature from measurements of Δ δH2O − δNAA or Δ δH2O − δTMA, if one were relying on calibration data obtained from noninfarcted tissue. The results of this study, however, imply that the chemical species comprising the in vivo NAA and TMA signal must not change sufficiently to produce a change in the chemical shift in the 1H MR peak, because there was no difference in the slope and intercept of the temperature-chemical shift difference relationship measured for noninfarcted and infarcted tissue. A potential exception to this conclusion may occur in the use of shorter TE (ie, TE < 136) for the collection of 1H MR spectroscopy data, because the relative contributions from other species, including macromolecules, is increased under these conditions (34, 39, 40).
Another factor that could limit the application of 1H MR spectroscopy temperature measurements in a clinical setting is that the variation in temperature readings suggests an accuracy of approximately ± 1°C, similar to that of our earlier studies (21, 22). As discussed previously, sources of error could include temperature variations within the voxel or during the MR spectroscopy data collection period, the digital resolution of data collection and phasing of spectra, and the temperature sensors themselves as well as the thermocouple used in the calibration (22). Because of these errors, it is difficult to draw firm conclusions regarding brain temperature variations during stroke or modulation of brain temperature responding to a cooling protocol from the study of individual patients when the change in brain temperature is in the range of 1° to 2°C.
Another concern is the reproducibility of measuring temperature changes between multiple MR sessions. Although not addressed directly by the present study, there are two lines of evidence to suggest that this is not a problem. First, data collected from different animals during a period of several weeks all follow the same linear relationship (Fig 3). Similar results were obtained in studies of swine brain (21, 22). Second, in a previous study of human subjects, there was no significant difference in brain temperature measured in two successive MR sessions, with and without localized head surface cooling (41). Therefore, temperature measures using this technique are fairly robust and independent of fluctuations in the MR system. The reason for this is that each MR spectrum has NAA or TMA as its own internal chemical shift reference.
Despite these reservations, we think that the 1H MR spectroscopy method described herein could help resolve a number of issues concerning brain temperature monitoring, manipulation, and regulation after stroke or other cerebral injuries. It remains unclear whether brain temperature is selectively regulated during fever associated with stroke, similar to that proposed in normal humans during heat stress (42). Alternatively, infarcted brain tissue temperature may be decreased substantially, similar to that reported as occurring in humans experiencing cerebral death (43). It is conceivable that significant temperature gradients could exist within the brain after stroke and that these gradients change as a function of evolving brain damage (19). A related issue is whether changes in core body temperature or temperature measured from other locations (ie, tympanum or oral) provide an accurate indication of changes in brain temperature after stroke. Finally, the efficiency of various protocols designed to reduce brain temperature therapeutically could be tested directly in human volunteers or in patients, as recently illustrated (41).
Conclusion
The primary finding of this study is that the relationship between temperature and the chemical-shift difference between water and the reference peaks of NAA or TMA (ie, Δ δH2O − δNAA or Δ δH2O − δTMA) do not differ between infarcted and non-infarcted brain tissue. Because these experiments were collected using a clinical MR imaging/MR spectroscopy system, the methodology is directly applicable to the study of human participants. Specifically, the results suggest that 1H MR spectroscopy could be used to obtain accurate measurements of brain temperature in humans after stroke for periods of up to at least 24 hours after insult. A second finding is that the relationship between brain temperature and the chemical-shift difference is the same regardless of whether a TE of 136 ms or 272 ms was used for 1H MR spectroscopy data collection.
Footnotes
↵1 Supported by the American Heart Association Texas Affiliate, National Institutes of Health Biotechnology Research Program (P41-PR02584), and the Departments of Pediatrics and Radiology.
↵2 Address reprint requests to Ronald Corbett, PhD, Ralph Rogers and Mary Nell Magnetic Resonance Center, Department of Radiology, University of Texas Southwestern Medical Center at Dallas, 5801 Forest Park Road, Dallas, TX 75235-9085.
References
- Received January 21, 1999.
- Copyright © American Society of Neuroradiology