Abstract
Summary: We describe a case of hereditary congenital mirror movements (MMs) in a 76-year-old man, who after an ischemic stroke, had persistence of MMs in the paretic hand during voluntary movements of the contralateral arm. By using functional MR imaging to investigate the performance of motor and sensory tasks with the affected and the unaffected hands, we found evidence for increased ipsilateral primary motor cortex activity and reduced transcallosal inhibition. Both these mechanisms are likely to be involved in the genesis of MMs.
Mirror movements (MMs) are involuntary movements on one side of the body occurring simultaneously to intentional movements of the homologous contralateral muscles. This phenomenon is usually observed in the distal parts of the extremities and is generally most marked in the hands. MMs occur physiologically in childhood and usually disappear with neurologic maturation (1, 2). Persistent MMs may occur in complex malformation syndromes, such as Kallmann syndrome, Klippel-Feil syndrome, or congenital hemiparesis or as an autosomal dominant trait. Among the mechanisms proposed to explain the occurrence of MMs, the most commonly accepted is an abnormally developed ipsilateral corticospinal pathway (3). Bilateral activity of the primary motor cortex interpreted as a sign of a lack of callosal inhibition has, however, been considered as an alternative or complementary explanation for MMs (3, 4).
We describe the case of congenital MMs in an adult subject who had a stroke affecting the left hemisphere, which caused a right hemiplegia, with preservation of the MMs in the affected upper limb. By investigating, with functional MR imaging (fMRI), the performance of tasks involving the motor and the sensory systems, we speculate that an increased activity of ipsilateral corticospinal projections and a reduced transcallosal inhibition both contribute to the presence of MMs.
Case Report
A right-handed 76-year-old man, with a history of hypertension and diabetes, was admitted to our institution for a sudden onset of a right-sided hemiplegia and severe hypoesthesia, associated with motor aphasia. CT of the brain performed on the same day of the acute episode showed a large hypoattenuated lesion in the left sylvian area involving the frontal, parietal, and opercular regions; the internal capsule; and basal ganglia. The lesion had microbleed components. Ultrasonography of the neck arteries showed diffuse subintimal thickness. Transthoracic and transesophageal echocardiography showed spontaneous echo contrast in the left atrium, and the electrocardiogram showed atrial fibrillation.
During clinical examination, voluntary movements of the left hand, such as gripping, caused MMs in the right hand, which was incapable of any intentional movement. Familial history of the patient revealed a hereditary congenital MM disorder, which also affected his brother, who died several years before the episode described herein. In both siblings, MMs were present only in the upper limbs and resulted in an interference of intermanual coordination and mild influence on activities of daily life. Familial and individual medical history excluded any other condition that could be associated with MMs.
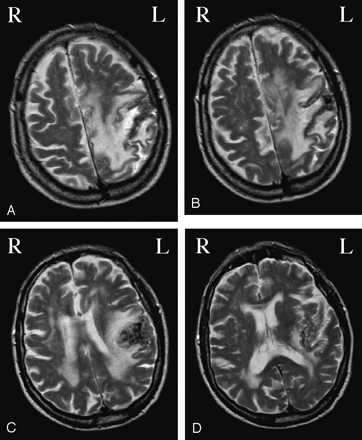
Two weeks after the stroke onset, the patient underwent an fMRI examination. During this period, there was no clinical recovery. Before the fMRI acquisition, a dual-echo sequence was acquired, which confirmed the presence of a large ischemic lesion in the left sylvian area with microbleed components, and diffuse white matter hyperintensities in periventricular and subcortical regions, with sparing of the corpus callosum (Figure 1).
Axial brain T2-weighted images of a patient with hereditary congenital MMs showing a large ischemic lesion in the left sylvian area, with microbleed components, and diffuse white matter hyperintensities in periventricular and subcortical regions.
The brain patterns of cortical activations following three different tasks were investigated using fMRI and a block design, where five periods of activation were alternated with six periods of rest (each period of activation and rest consisting of five measurements). Task 1 consisted of repetitive flexion-extension of the last four fingers of the left hand moving together. During this task, MM of the right paretic hand was observed. Tasks 2 and 3 consisted of tactile stimulation (palm brushing) of the palm of the left and right hands, respectively. The tactile stimulation was performed by an observer that remained in the imager room during the entire fMRI acquisition. This observer received visual commands by another observer located in the MR console room. Analysis of fMRI data was performed using statistical parametric mapping (SPM99), as described elsewhere (5). We report activations below a threshold of P = .05, corrected for multiple comparisons.
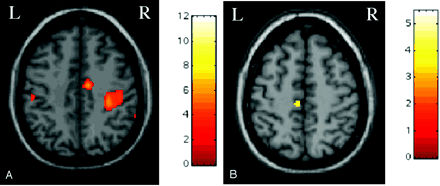
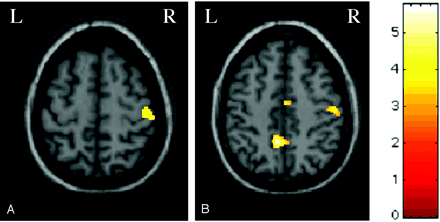
During task 1, significant activations were detected in the right and left primary sensorimotor cortices (SMC); right supplementary motor area (SMA); cerebellar hemispheres, bilaterally; right basal ganglia; and right intraparietal sulcus (Figure 2). A significant deactivation of the left SMA was also detected (Figure 2). During task 2, significant activations were detected in the right primary SMC (Figure 3) and secondary sensorimotor cortex (SII). Finally, during task 3, significant activations were detected in the right primary SMC and SMA (Figure 3), in the left precuneus and cuneus and in the SII, bilaterally.
Color-encoded brain activations superimposed on a high-resolution T1-weighted image in the standard SPM space in a patient with congenital MMs during left hand movement (task 1). A, Clusters of activation are visible for the right and left primary SMC and right SMA. B, Cluster of deactivation is visible for the left SMA.
Color-encoded brain activations superimposed on a high-resolution T1-weighted image in the standard SPM space in a patient with congenital MMs during left (A) (task 2) and right (B) (task 3) hand tactile stimulation. A, Cluster of activation is visible for the right primary SMC. B, Clusters of activation are visible for the right primary SMC and SMA and for the left precuneus.
Discussion
MMs are normally present in the first phases of human motor development and have been related to the activity of ipsilateral projections from the motor cortex to spinal motoneurons innervating the skeletal muscles (1). Their disappearance with neurologic maturation has been interpreted as a result of maturational changes, mainly involving callosal inhibition (1). Conversely, MM persistence in adulthood has been suggested to be the consequence of an impaired interhemispheric inhibition or of an abnormal corticospinal tract development (2). Neurophysiologic (6), positron-emission tomographic (6), and neuroradiologic (7) data support the role of an abnormal ipsilateral corticospinal tract development in the genesis of MMs, especially in case of developmental disorders (7). The potential contribution to the genesis of MMs of an impaired transcallosal inhibition, with a subsequent bilateral activation of the primary motor cortex, has also been suggested by studies by using movement-related cortical potentials (4) and fMRI (3).
The present case represents the first fMRI report on the effect of an acquired acute brain lesion on congenital MMs and might serve as a valuable natural “lesion” model to improve our understanding of the pathophysiology of MMs. The activity of the left primary SMC mediated through the corticospinal and the thalamo-cortical tracts was likely to be severely damaged by the large ischemic lesion that caused the stroke, as also shown by the complete absence of any contralateral SMC activation during the performance of task 3 (tactile stimulation of the right hand). During the performance of this task, however, activation of the right primary SMC and of the SII (bilaterally) was found. Although the activation of the ipsilateral primary S1 and SII following tactile stimulation is less well documented than that of ipsilateral M1 during a motor task, there are a few reports of such a finding in healthy subjects and in patients with different neurologic conditions, which suggests that this event might be secondary to the activity of transcallosal pathways (8, 9). Although this single-subject report cannot establish whether the observed altered transcallosal activity simply reflects congenital MMs or is modulated by the ischemic lesion, our findings support the notion of an altered transcallosal inhibition in the genesis of MMs. During the performance of task 1, a simple motor task with the unaffected hand, which was associated with an MM of the paretic hand, we found a bilateral activation of the primary SMC. Because the crossed corticospinal projections between the left primary SMC and spinal motoneurons innervating the right hand is unlikely to be responsible for this activation, altered transcallosal inhibition might again be considered as an alternative explanation for the activity of the left primary SMC. Inter- and intrahemispheric connections between the primary SMCs are thought to be mediated primarily by the SMA. This fits with the observation that during task 1 the right SMA was activated, whereas the left SMA was deactivated, which suggests an altered functional connection between each primary SMC and the corresponding SMA. The identification of a blood oxygenation level effect in the left primary SMC and SMA despite the extensive infarct suggests that these regions are not completely damaged by the lesion, although their activities might be impaired by the accompanying edema.
Increased activation of the ipsilateral primary SMC has also been described in stroke patients with MMs in the unaffected limb during voluntary movements of the affected one (10). Because an fMRI examination of this patient before the stroke is unavailable, we are not able to define whether the ischemic lesion might have affected the brain pattern of cortical activations. The pattern of MMs in our patient, however, differed from that reported in stroke patients without previous evidence of MMs (10), because in our patients MMs occurred in the affected hand during the movement of the unaffected one. Therefore, it is most likely that the observed activations reflect those related to congenital MMs and not those secondary to recent damage.
Conclusion
Although the results of this study are based on the assessment of a single subject at a single time, they suggest that transcallosal inhibition and an alteration of ipsilateral primary motor cortex activity are likely to cause the persistence of MMs. Whereas the large ischemic lesion provided a unique model to investigate the nature of MMs in adults, it also limited our ability to disentangle the effect of such a lesion and that associated to MM persistence on the observed pattern of activations.
References
- Received May 28, 2004.
- Accepted after revision July 12, 2004.
- American Society of Neuroradiology