Abstract
BACKGROUND AND PURPOSE: Postoperative contrast-enhanced MR imaging of the brain is routinely used when evaluating for residual or recurrent brain tumor. It is imperative to be aware of morphologic changes and imaging features that typically occur in response to surgical manipulation at the postoperative site to avoid misinterpretation of imaging findings. Our purpose was to determine normal postoperative changes and alterations in the choroid plexus among patients who had undergone temporal lobectomy in order to distinguish this appearance from pathologic changes that may be seen in the presence of infection or recurrent tumors.
METHODS: We reviewed 159 MR scans from 95 patients with hippocampal sclerosis or gliosis who underwent temporal lobectomy for treatment of intractable epilepsy. Choroid plexus location and size were assessed on contrast-enhanced T1-weighted images.
RESULTS: After temporal lobectomy, the choroid plexus enlarged and sagged into the resection site. Increase in the size of the choroid plexus occurred in 58% of cases overall. The degree of enhancement also increased after surgery, sometimes resulting in a nodular pattern of enhancement. The changes were most marked during the 1st week after temporal lobectomy, and showed an enlarged, markedly enhancing choroid plexus on 86% of the scans.
CONCLUSION: Postoperative changes of the choroid plexus after temporal lobectomy include sagging into the resection site, an increased size, and an increased degree of enhancement. Normal postoperative morphologic characteristics may mimic neoplastic enhancement pattern. Familiarity with this appearance is important to avoid a pitfall in diagnosis of recurrent postoperative temporal lobe neoplasms.
Postoperative contrast enhanced MR imaging of the brain is routinely used when evaluating for residual or recurrent brain tumor (1, 2). It is imperative to be aware of the morphologic alterations and imaging features that typically occur in response to surgical manipulation at the postoperative site in order to avoid a misinterpretation of imaging findings. Several studies have demonstrated that contrast enhancement of the surgical margin occurs “normally” during the 1st month after surgery and may be seen as early as the first postoperative day (1, 3–5). These observations have important implications for patients undergoing surgery for neoplastic lesions. A nodular pattern of enhancement that mimics tumor enhancement can occur at the surgical margins 5 days after surgery and last for a variable period of time. Therefore, the optimal window for imaging such a patient is in the first 4 days after surgery.
Although the normal postoperative enhancement pattern has been reported for the parenchymal surgical margins by several authors, choroid plexus changes occurring sequentially after temporal lobectomy have not been described. The purpose of this study was to investigate the normal changes occurring postoperatively after temporal lobectomy. These findings are useful for patients with neoplasm or infections in order to distinguish normal postoperative changes from those attributable to pathologic processes. The study population consisted of patients undergoing surgery for seizure control not related to neoplastic, inflammatory, or vascular lesions.
Methods
All patients underwent temporal lobectomy between 1987 and 1995. In order to detect contrast enhancement changes attributable solely to surgery, patients with neoplasms or enhancing lesions on preoperative MR scans were excluded from the study. All patients had a histologic diagnosis of hippocampal sclerosis, hippocampal gliosis, or normal tissue. Patients with infectious complications at the surgical site were also excluded. Ninety-five subjects from whom 159 postoperative contrast-enhanced MR scans were performed comprised the study group. Their ages ranged from 9 to 68 years, with a mean age of 32 years. Of these, 54 patients underwent only one postoperative MR examination, whereas the remaining 41 had serial postoperative MR examinations. The interval between surgery and imaging ranged from 9 hours to 5.6 years.
All MR examinations were performed on a 1.5-T unit (Signa; GE Medical Systems, Milwaukee, WI). Both T1-weighted coronal and T2-weighted axial images were obtained before administration of contrast material. T1-weighted coronal images were obtained after IV administration of 0.1 mmol/kg gadopentetate dimeglumine (Magnevist, Berlex, Wayne, NJ). In addition, studies were supplemented with axial or sagittal contrast-enhanced T1-weighted images in most cases. The parameters for the T1-weighted coronal spin-echo series were 400–750/10–30 (TR/TE); one to four signals; section thickness, 5 mm; field of view, 20 to 25 cm; and matrix size, 192 × 256 or 128 × 256. Spin-echo T2-weighted axial image parameters were 2000–2500/80–100; one to two signals; section thickness, 5 mm; field of view, 20 to 25 cm; matrix, 192 × 256 or 256 × 256.
The size, morphologic characteristics, and degree of enhancement of the choroid plexus on the postoperative coronal images were evaluated. The postoperative choroid plexus was classified as either normal or abnormally prominent (with respect to size enlargement and an increased degree of enhancement) as compared with the contralateral side that was not resected.
Results
The pathologic diagnosis was hippocampal sclerosis in 83 patients. Nonspecific gliosis was found in the hippocampus in 11 patients, and no histologic abnormality was discovered in a single patient.
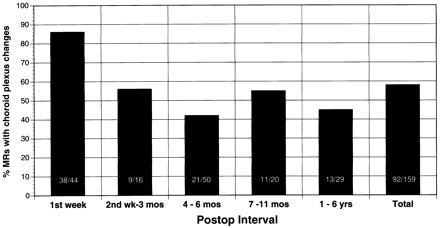
After temporal lobectomy, the size and degree of enhancement of the choroid plexus on contrast-enhanced images was increased compared with the contralateral, uninvolved side (Figs 1 and 2). Ipsilateral choroid plexus prominence was noted in 58% (92/159) postoperative MR scans. In the 1st week after surgery, choroid plexus prominence occurred in 86% (38/44) MR scans. Between 2 weeks and 6 years after surgery, such prominence was seen on about half the MR scans (Fig 3). The enhancement pattern was sometimes nodular, which could potentially mimic the nodular appearance of recurrent or residual neoplasm occurring at the surgical margin (Figs 1, 2, and 4). A finding associated with choroids plexus prominence was the sagging of the choroid plexus into the surgical bed (Fig 5).
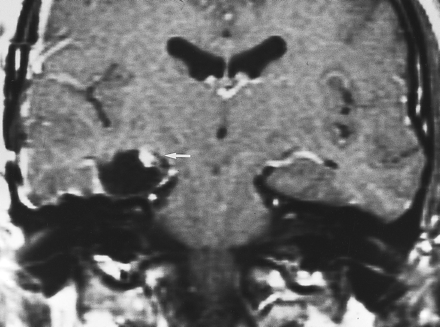
Typical postoperative changes of the choroid plexus after temporal lobectomy. Coronal postcontrast T1-weighted MR image, obtained in the immediate postoperative period (1 day) after right temporal lobectomy, shows enlargement and intense nodular enhancement of the choroid plexus on the right (arrow) compared with the normal side
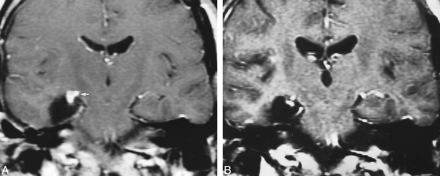
Postoperative changes of choroid plexus with time, on enhanced T1-weighted coronal images. A, Intense, nodular enhancement of the choroid plexus (arrow) on the right side in the immediate (1 day) postoperative period. B, Persistent nodular changes 9 months after surgery, although the enlargement and the degree of enhancement have markedly diminished in the interval
This graph illustrates the frequency of the choroid plexus changes as a function of time. Thirty-eight (86%) of the 44 MR scans performed within the 1st week after surgery revealed an enlarged, intensely enhancing choroid plexus. These choroid plexus changes persist in about half the cases after the 1st postoperative week
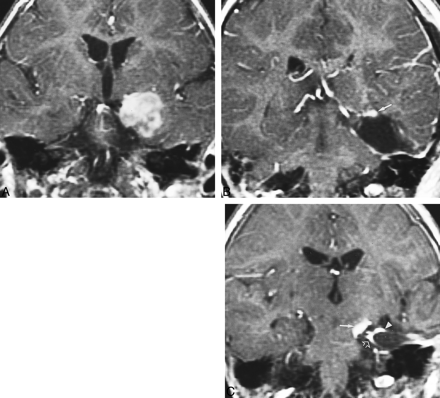
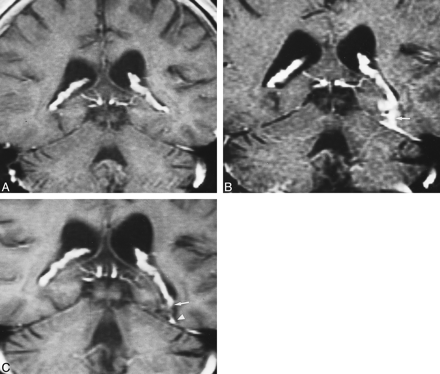
Recurrent neoplastic disease with similar appearance to postoperative choroid plexus enhancement revealed on coronal enhanced T1-weighted images. A, The preoperative image shows a large, heterogeneously enhancing tumor involving the medial temporal lobe. B, MR scan 1 month after resective surgery shows a prolapsed choroid plexus adherent to the surgical margins (arrow). C, At 3 months after surgery, several enhancing foci are present. Recurrent enhancing oligodendroglioma was found medially (arrow), as confirmed on sequential images (not seen). The choroid plexus enhancement (arrowhead) adjacent to the tumor as well as the dural enhancement (open arrows) could be misinterpreted as neoplastic enhancement.
Postsurgical choroid plexus herniation on coronal, enhanced, T1-weighted images. A, Preoperative scan shows the normal-appearing choroid plexus in the lateral ventricles. B, Immediate postoperative scan depicts herniation of the choroid plexus inferiorly into the surgical site (arrow). C, Persistent sagging of the choroid plexus 6 months (arrow) after surgery. This should be distinguished from adjacent postoperative dural enhancement (arrowhead).
Discussion
Several reports note the importance of observing nodular enhancement at the postoperative site with MR imaging. Albert et al (1) evaluated MR images from 60 patients after resection for malignant glioma. Approximately 80% of tumor recurrences were seen to arise from prior enhancing “nodular” or “masslike” lesions, which were retrospectively recognizable on MR examinations performed 1 to 4 days after surgery. However, a nodular pattern of enhancement along the surgical margins was also observed by Sato et al (3) among patients who underwent epilepsy surgery for benign disease. This occurred during the period of peak enhancement, ie, 1 week to 1 month postoperatively. Therefore, it appears that a nodular enhancement represents part of the spectrum of the “normal” postoperative appearance. Such a pattern of enhancement, if seen postoperatively in a patient having undergone surgery for malignant disease, could be mistaken for residual or recurrent tumor.
In this study, enlargement of the choroid plexus was seen in 86% of the cases in the 1st postoperative week. A nodular pattern of enhancement early in the postoperative period was also commonly seen. This could potentially mimic residual/recurrent tumor. Familiarity with the altered postoperative anatomy will help to avoid this pitfall in diagnosis—the enhancing nodule is always contiguous with the choroid plexus on the side of the surgery.
In order to understand the etiology of these postoperative changes, it is helpful to review the surgical technique for temporal lobectomy. At our institution, an anteromedial temporal lobectomy involves an en bloc surgical resection of the amygdala, hippocampus, uncus, and parahippocampal and fusiform gyri (6). The temporal horn is opened, and an incision is made inferiorly through the horn to the floor of the middle cranial fossa, just lateral to the fusiform gyrus. This incision is then extended posteriorly into the atrium of the lateral ventricle. This deficiency in the floor of the temporal horn and atrium allows varying degrees of herniation of the choroid plexus through it, resulting in the sagging observed in our cases. The profuse enhancement of the choroid plexus is because of its highly vascular structure and lack of a blood-brain barrier. Inflammatory changes postoperatively cause further hyperemia and pronounced enhancement. It may also be related to retraction injury during the surgical procedure, particularly with radical hippocampectomy. During this procedure, more forceful retraction is needed in order to resect the distal end of the hippocampus at the posterior edge of the brain stem. The free edge of the sagging choroid plexus may be clumped together, resulting in the nodularity observed in some cases. If this sagging choroid plexus becomes adherent to the margin of the resection site, a markedly enhancing nodular appearance may result. The choroid plexus changes are most prominent in the 1st week, probably because of tissue injury associated with surgical resection (3).
In conclusion, it is important to be familiar with normal postoperative imaging findings. The use of a surrogate population, ie, those who have undergone epilepsy surgery, allows us to study enhancement of the human brain without the confounding problem of discriminating tumor from nontumor enhancement (3, 5, 7). The findings of the current study have important implications with respect to MR imaging findings in a patient with a neoplastic condition.
Footnotes
1 Address reprint requests to Richard A. Bronen, M.D., Department of Diagnostic Radiology, Yale University School of Medicine, P.O. Box 208042, New Haven, CT 06520-8042.
- Received November 18, 1999.
- Accepted after revision April 19, 2000.
- Copyright © American Society of Neuroradiology