Abstract
BACKGROUND: Mild traumatic brain injury is theorized to cause widespread functional changes to the brain. Resting-state fMRI may be able to measure functional connectivity changes after traumatic brain injury, but resting-state fMRI studies are heterogeneous, using numerous techniques to study ROIs across various resting-state networks.
PURPOSE: We systematically reviewed the literature to ascertain whether adult patients who have experienced mild traumatic brain injury show consistent functional connectivity changes on resting-state -fMRI, compared with healthy patients.
DATA SOURCES: We used 5 databases (PubMed, EMBASE, Cochrane Central, Scopus, Web of Science).
STUDY SELECTION: Five databases (PubMed, EMBASE, Cochrane Central, Scopus, and Web of Science) were searched for research published since 2010. Search strategies used keywords of “functional MR imaging” and “mild traumatic brain injury” as well as related terms. All results were screened at the abstract and title levels by 4 reviewers according to predefined inclusion and exclusion criteria. For full-text inclusion, each study was evaluated independently by 2 reviewers, with discordant screening settled by consensus.
DATA ANALYSIS: Data regarding article characteristics, cohort demographics, fMRI scan parameters, data analysis processing software, atlas used, data characteristics, and statistical analysis information were extracted.
DATA SYNTHESIS: Across 66 studies, 80 areas were analyzed 239 times for at least 1 time point, most commonly using independent component analysis. The most analyzed areas and networks were the whole brain, the default mode network, and the salience network. Reported functional connectivity changes varied, though there may be a slight trend toward decreased whole-brain functional connectivity within 1 month of traumatic brain injury and there may be differences based on the time since injury.
LIMITATIONS: Studies of military, sports-related traumatic brain injury, and pediatric patients were excluded. Due to the high number of relevant studies and data heterogeneity, we could not be as granular in the analysis as we would have liked.
CONCLUSIONS: Reported functional connectivity changes varied, even within the same region and network, at least partially reflecting differences in technical parameters, preprocessing software, and analysis methods as well as probable differences in individual injury. There is a need for novel rs-fMRI techniques that better capture subject-specific functional connectivity changes.
ABBREVIATIONS:
- DMN
- default mode network
- FC
- functional connectivity
- ICA
- independent component analysis
- IQR
- interquartile range
- mTBI
- mild traumatic brain injury
- rs-fMRI
- resting-state fMRI
- SN
- salience network
- TBI
- traumatic brain injury
Mild traumatic brain injury (mTBI) is a common injury that, nevertheless, can pose difficult diagnostic and therapeutic challenges.1 Conventional neuroimaging including CT and structural MR imaging plays a key role in mTBI assessment and management, such as the identification of intracranial hemorrhage, but it has limited sensitivity for the detection of underlying abnormalities that have no clear macrostructural correlate.2 Advanced neuroimaging has been used during the past decade in an attempt to characterize more subtle post-mTBI neurobiological changes.
After mTBI, it is believed that disruptions occur in the organization of large-scale brain activity.2 Blood oxygen level–dependent functional MR imaging has, thus, been used to study changes in intrinsic brain connectivity. In particular, functional connectivity (FC) as reflected in low-frequency blood oxygen level–dependent fluctuations of resting-state fMRI (rs-fMRI),3 and its organization into various resting-state networks1,4 has been used in an attempt to understand the injury.
Approximately a decade ago, a comprehensive review of fMRI in mTBI by McDonald et al1 included a mere 2 studies of resting-state changes following mTBI. Since then, there has been an explosion of literature in this area. Part of the challenge in interpreting these results is the diversity of brain regions and networks studied and the great variety of methods that can be used to analyze FC in rs-fMRI. Common approaches include correlational methods (relying on selections of ROIs and/or seeds and correlating the corresponding rs-fMRI signal time-series with time-series of all other voxels [ROI/seed-to-voxel] or other ROIs/seeds [ROI/seed-to-ROI/seed] to map connectivity5) as well as independent component analysis (ICA; decomposition of brain-wide rs-fMRI into independent spatiotemporal components that can be correlated to determine connectivity6), both of which may be performed in a static fashion using the entire time-series or in a dynamic fashion using a sliding-window approach. Graph theory can also be used to study FC at either local or global levels.7 Finally, regional homogeneity is commonly used to measure synchronization of low-frequency fluctuations of a particular voxel with its nearest neighbors,8 while fractional amplitude of low-frequency fluctuations quantifies low-frequency oscillations as a reflection of local spontaneous activity.9
In this systematic review, we explore the current literature on FC in mTBI regarding whether adult patients who have mTBI show consistent FC changes on rs-fMRI, compared with healthy patients. We summarize thematic findings in terms of FC changes following injury and comment on discordances that are observed.
MATERIALS AND METHODS
Database Search
This study was registered with the International Prospective Register of Systematic Reviews (PROSPERO; ID CRD42022360114) and performed as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy (PRISMA) guidelines.
In November 2022, five databases (“PubMed,” “EMBASE,” “Cochrane Central,” “Scopus,” “Web of Science”) were searched for research published since 2010. The search strategy used the keywords “functional MR imaging” and “mild traumatic brain injury” as well as related terms. Search terms can be found in the Online Supplemental Data.
Inclusion criteria were the following: peer-reviewed human research 1) performed in adults older than 18 year of age; 2) written in English; 3) involving the use of rs-fMRI; 4) including a measure of FC; 5) published in or after 2010; and 6) comparing patients with mTBI with healthy controls. Blast/military traumatic brain injury (TBI) was excluded because military-related blast injury has a unique mechanism relating to propagating pressure waves. Sport- and athletic-related injury was also excluded because many such studies occur either in pediatric subjects and/or specifically feature individuals exposed to repetitive head impacts and repeat TBI. Of course, many studies may have included a number of these types of etiologies without distinguishing their results from those of other etiologies. Consequently, we were able to exclude only studies whose entire cohort had one of these mechanisms. Specifically, exclusion criteria were the following: 1) preclinical animal studies; 2) inclusion of pediatric patients younger than 18 years of age; 3) reviews, meta-analyses, books, case reports, or case series; 4) moderate or severe TBI; 5) cohorts of only sports- or athletic-related TBI; 6) cohorts of only blast-related TBI; 7) cohorts of only military TBI; 8) cohorts of only repetitive TBI; and 9) only task-mediated fMRI studies.
All results were screened at the abstract and title levels by 4 reviewers (S.D., S.A., J.W., L.S.) according to predefined inclusion and exclusion criteria. Each study was evaluated independently by 2 reviewers, with discordant screening settled by consensus. Two reviewers (S.D., S.A.) performed full-text data screening and extraction, with disagreements settled by consensus.
Data Extraction and Synthesis
We excluded the following categories: article characteristics (title, author, journal, year), cohort demographics (time since injury, age, sex), fMRI scan parameters (ie, TE, TR, scan duration, scanner model, and magnet strength), data analysis processing software, atlas used, data characteristics (including analysis method applied, studied ROI or network, connectivity measures), and statistical analysis information (method, multiple-comparison correction).
FC changes were graded on an ordinal scale from −2 to +2: −2 and +2 denoting studies reporting only decreases or increases in FC, respectively; −1 and +1 denoting studies reporting mixed results with most areas showing decreased or increased FC, respectively; and 0 if no changes were reported. If there were an equal number of areas with increased and decreased changes, we assigned an ordinal score of 0 but noted that there was equal change in each direction.
For all studies, time since mTBI was binned into either <1 month, 1–6 months, or >6 months, with some studies having multiple evaluations at different time points. For these studies, results were examined separately for each of these time periods.
Of note, in some cases, the whole brain was evaluated by ICA, and several resting-state networks were subsequently identified as independent components. In most such cases, the identified components were specific named resting-state networks; for these studies, we report the individual networks and their changes, as well as the whole brain and its change as a conglomeration of the network changes.
Risk of bias and applicability were assessed by 3 reviewers (S.D., S.A., J.W.). Each study was evaluated by 2 reviewers with disagreements settled by consensus. The case-control version of Newcastle-Ottawa Scale was used for this assessment.10
RESULTS
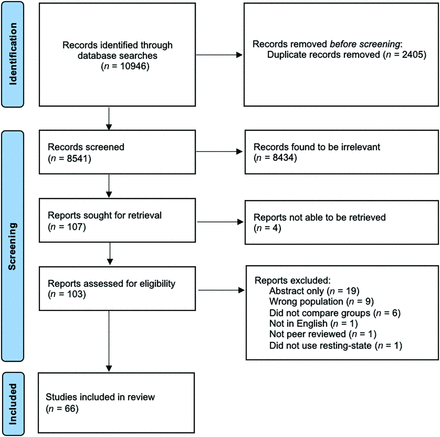
Our initial search found 10,946 results, 2405 of which were duplicates and were removed before screening. The remaining 8541 underwent title and abstract screening from which 107 results were considered for full-text review. Four of these could not be found online and may have been abstracts or conference papers. Among the 103 remaining results, 19 were only abstracts and 18 did not meet the criteria for inclusion (9 studied the wrong population, 6 did not compare with a healthy control group, 1 was not in English, 1 was not peer-reviewed, and 1 was not an rs-fMRI study). Full-text data extraction was ultimately performed for 66 studies (Fig 1).2,4,8,11-73 A summary of these articles can be found in the Online Supplemental Data.
PRISMA flow diagram detailing study identification, screening, and inclusion.
Risk of Bias
The case-control version of the Newcastle-Ottawa Scale was used for assessment of the risk of bias. Representativeness of cases for Shumskaya et al70 was thought to have unclear applicability because only fronto-occipital injuries were included. Otherwise, there was appropriate selection, comparability, and exposure for all studies, because subjects with and without mTBI were clearly distinguishable and verified through interview.
Cohort Characteristics and Technical Parameters
These studies included a median of 31 healthy controls (interquartile range [IQR], 20–42). Depending on the time since mTBI was evaluated in the study, studies with subjects scanned within 1 month of mTBI included a median of 48 (IQR, 28.5–56.5) subjects, studies with subjects scanned between 1 and 6 months of mTBI included a median of 25 (IQR, 23–42) subjects, and studies with subjects scanned >6 months from mTBI included a median of 28 (IQR, 20.5–50) subjects. Thirty-eight of 66 (57.6%) studies did not specify the mechanisms of mTBI. Among the 28 that did, traffic/motor vehicle collisions were by far the most common etiology (18/28, 64.3%).
On average, the healthy control cohort consisted of a mean of 47.6% women (SD, 12.6%) with a mean age of 36.6 (SD, 6.7) years, and the mTBI cohort consisted of 43.6% women (SD, 14.3%) with a mean age of 36.6 (SD, 7.0) years.
Regarding the MR imaging scanner, 60 studies (90.9%) used a 3T magnet, 5 studies (7.6%) used a 1.5T magnet, and one (1.5%) did not specify the magnet strength. The mean TE and TR were 29.1 (SD, 4.4 ) ms and 2082.1 (SD, 308) ms. The mean rs-fMRI duration was 7.4 (SD, 2.5) minutes.
Processing software used across all the studies varied from statistical parametric mapping (SPM, Versions 5, 6, 8, 12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12), FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl), Group ICA of fMRI Toolbox Software (GIFT; http://mialab.mrn.org/software/gift/), Analysis of Functional NeuroImages (AFNI; http://afni.nimh.nih.gov/), medInria (https://med.inria.fr/), Data Processing & Analysis of Brain Imaging (DPABI; http://rfmri.org/DPABI), FreeSurfer (https://surfer.nmr.mgh.harvard.edu/fswiki/DownloadAndInstall), the graph theoretical network analysis (GRETNA; https://www.nitrc.org/projects/gretna/) toolbox, to the Resting-State fMRI Data Analysis Toolkit (REST; http://rfmri.org/REST).
Connectivity Changes
Across 66 studies, 80 distinct areas were collectively analyzed 239 times at least at 1 time point (Online Supplemental Data). Sixteen analyses included multiple time points (<1 month between mTBI and the scan, 1–6 months, and >6 months) (Online Supplemental Data). The most common analysis approaches used were ICA (n = 106), ROI/seed-to-ROI/seed (n = 62), and ROI/seed-to-voxel (n = 32), followed distantly by graph theory methods (n = 17, Online Supplemental Data), ICA-based dynamic FC (n = 14), regional homogeneity (n = 4), and fractional amplitude of low-frequency fluctuations (n = 4).
The most-commonly analyzed areas and networks were the whole brain (45 analyses across 37 studies), the default mode network (DMN) (32 analyses across 25 studies), and the salience network (SN, 13 analyses across 10 studies). Studies that separately analyzed patients with and without postconcussive symptoms are explicitly labeled in Supplementary Table 2 (Online Supplementary Data).
Figure 2 shows panels of histograms of FC changes in the whole brain, the DMN, and the SN, with respect to scans performed <1 month since mTBI, 1–6 months since mTBI, and >6 months since mTBI. By comparing the left half of each plot (frequencies of −2 and −1) with the right half (frequencies of 1 and 2), we can get a sense of the relative frequency of decreased-versus-increased FC. For example, <1 month after mTBI, there were 9 more analyses that found decreased FC across the whole brain compared with increased FC (7, −2 studies; 8, −1 study; 1, +1 study; and 5, +2 studies), possibly suggesting a trend toward decreased whole-brain FC acutely after mTBI. These changes may persist 1–6 months post-mTBI in the whole brain, whereas there are 5 studies that found decreased FC versus none with increased FC. At >6 months in the whole brain, as well as all time points with the DMN and SN, the differences are not as robust.
FC changes, categorized on an ordinal scale and separated by the time since injury. −2 = only decreased FC changes, −1 = more areas of decreased FC change than areas of increased FC change, 0 = no FC changes (black) or the same number of areas of decreased and increased FC (gray), 1 = more areas of increased FC change, 2 = only areas of increased FC changes (changes all relative to results in healthy controls). Gray bars are separate from black bars, ie, 3 studies found an equal number of areas of increased and decreased FC in the DMN at <1 month. Eight found no changes, so a total of 11 studies were classified as a 0.
DISCUSSION
In the past decade, many more approaches to studying mTBI using rs-fMRI have been developed and applied. The 2012 review of McDonald et al1 found only 2 studies evaluating the effect of mTBI on resting-state FC, one of which looked at the thalamus with ROI-to-voxel and ICA approaches and another that looked at the DMN and the task-related network with an ROI-to-ROI approach.4,72 In comparison, this present review includes 66 studies using any of 9 analysis methods to study a total of 61 distinct areas, demonstrating how much the literature has grown in the interim.
In part, because of this growth, the data we reviewed show immense heterogeneity with respect to FC changes, even among studies that evaluate the same networks or ROIs using the same techniques in the same timeframe since injury. Most reassuring, control and mTBI groups, on average, were similar in both age and sex distribution, and nearly all studies were performed on 3T magnets. Besides variability in methods as noted above, probably one of the most challenging aspects of studying mTBI arises from the heterogeneity of the injury itself and the manifestations of injury. This issue continues to plague the field because injured individuals are inherently difficult to categorize. In addition, another source of variability among studies likely arises from technical sources such as scan parameters, preprocessing methods, brain atlas selected, and so forth.
When analyzing FC changes, we focused on 3 main regions: the whole brain, DMN, and SN, because these were the most widely studied. There was a slight predilection toward decreased whole-brain FC early after mTBI (<1 month of injury), though the heterogeneity of the results precludes drawing any strong conclusion. Also, there was no definite trend across time, though more studies showed decreased FC within 1 month of injury compared with 1–6 months or >6 months after injury, suggesting that perhaps functional hypoconnectivity is the dominant response immediately following mTBI and is followed by recovery with time.
The underlying neurophysiologic changes following mTBI are not yet fully characterized, so it is difficult to confidently identify a biological basis for acute hypoconnectivity. Increasing evidence points to cerebrovascular injury as a key hallmark of mTBI, characterized by disrupted cerebrovascular reactivity and neurovascular coupling.74 Studies using arterial spin-labeling to quantify CBF after mTBI report inconsistent findings, with suggested reduced CBF acutely after injury and varied responses in the subacute phase, similar to our findings regarding FC after mTBI.75 Because cerebrovascular disease is known to influence network connectivity, there may be a link between FC changes and CBF changes.76 In any case, it is clearly possible that there is a temporal evolution of connectivity changes across time after injury, and this is reflected in the literature.
Limitations include exclusion of studies consisting entirely of military, sports-related mTBI, and pediatric patients. Moreover, some studies included a small number of participants with sports-related mTBI; we were unable to exclude FC changes from only these specific subjects, so they do contribute to our results. Second, due to the high data heterogeneity, we could not be as granular as we would have liked. For example, we were forced to bin FC changes into arbitrary temporal categories, which likely limit our view of temporal changes after injury. It is certainly possible that there are more nuanced temporal changes within or across these bins that were not feasible to capture; and, in fact, the current results suggest that this possibility may be true. Similarly, for the sake of practicality, we present FC changes on an ordinal scale based on the number of regions within each study found to show FC changes; however, this particular metric may not well-capture FC changes in mTBI. We also were not able to report the location of FC changes due to the amount of data analyzed. Finally, we did not perform subgroup analyses on patients with persistent symptoms, though the Online Supplementary Data do include information on studies that looked at ROIs/networks in asymptomatic-versus-symptomatic patients. We hope that our conglomerated data summary makes it easier for future investigations to identify and analyze subsets of these studies to answer more focused questions.
CONCLUSIONS
rs-fMRI is a noninvasive method to study FC of the brain that has been increasingly applied over the past decade to understand underlying functional brain alterations after mTBI. Due to a variegated landscape of rs-fMRI analysis methods and the still relatively naive understanding we have of mTBI, we find immense heterogeneity in the literature. We see a slight tendency toward decreased whole-brain FC within 1 month of mTBI, though group-based fMRI analysis at the present time does not easily reveal clear concordance among published studies. This issue may relate to a combination of underlying heterogeneity in the mTBI population as well as current limitations of rs-fMRI group-wise analysis methods. As a result, the present study is also limited in its ability to parse temporal differences and nuanced changes in connectivity across studies. There is a need for an improved description of subjects with mTBI as well as new rs-fMRI approaches that better capture subject-specific alterations of brain connectivity.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received October 30, 2023.
- Accepted after revision January 22, 2024.
- © 2024 by American Journal of Neuroradiology