Abstract
BACKGROUND AND PURPOSE: The hypoperfusion intensity ratio is a surrogate marker for collateral status and a predictor of infarct growth, malignant cerebral edema, and hemorrhagic transformation. Its utility to predict a poor NIHSS score and early neurologic deterioration after mechanical thrombectomy for large vessel (LVO) versus distal and medium vessel occlusions (DMVO) has not been investigated. The objective of this study was to determine whether the higher hypoperfusion intensity ratio is associated with a worse NIHSS score at 24 hours post-mechanical thrombectomy and early neurologic deterioration in LVO versus DMVO acute ischemic stroke.
MATERIALS AND METHODS: This was a retrospective study of 231 patients with acute ischemic stroke with LVO or DMVO amenable for mechanical thrombectomy and available CTP for hypoperfusion intensity ratio assessment pre-mechanical thrombectomy. Clinical and imaging characteristics were abstracted from the medical records. The primary outcome was the NIHSS score at 24 hours post-mechanical thrombectomy. The secondary outcome was early neurologic deterioration, defined as a >4-point increase in the NIHSS score between the initial assessment and 24 hours post-mechanical thrombectomy. All analyses were first conducted in the entire cohort and then separately for the LVO versus DMVO groups.
RESULTS: The optimal hypoperfusion intensity ratio threshold to detect early neurologic deterioration was 0.54. A hypoperfusion intensity ratio ≥ 0.54 was more frequently present in LVO versus DMVO (n = 37 [77.1%] versus n = 11 [22.9%]; P < .001). On multivariable linear regression, the hypoperfusion intensity ratio ≥ 0.54 was independently associated with a worse NIHSS score at 24 hours post-mechanical thrombectomy in the entire cohort (β = 0.163; P = .002) and the LVO group (β = 0.210; P = .005), but not in the DMVO group. The early neurologic deterioration occurred in 26 (11.3%) subjects. On multivariable logistic regression, there was no association of the hypoperfusion intensity ratio ≥ 0.54 with early neurologic deterioration in the entire cohort. However, when analyzed separately, a hypoperfusion intensity ratio ≥ 0.54 significantly increased the odds of early neurologic deterioration in subjects with LVO (OR = 5.263; 95% CI, 1.170–23.674; P = .030) but not in the DMVO group.
CONCLUSIONS: The hypoperfusion intensity ratio ≥ 0.54 was independently associated with a worse 24-hour post-mechanical thrombectomy NIHSS score and early neurologic deterioration in LVO, but not in DMVO acute ischemic stroke. Pending confirmation in future, prospective studies assessing the hypoperfusion intensity ratio may help identify patients at risk of secondary decline to improve peri-thrombectomy care and clinical decision-making.
ABBREVIATIONS:
- AIS
- acute ischemic stroke
- DMVO
- distal and medium vessel occlusion
- END
- early neurologic deterioration
- ENI
- early neurologic improvement
- HIR
- hypoperfusion intensity ratio
- LA
- leukoaraiosis
- LKW
- last known well
- LVO
- large vessel occlusion
- MT
- mechanical thrombectomy
- PCA
- posterior cerebral artery
- rCBF
- relative CBF
- SBP
- systolic blood pressure
- Tmax
- time-to-maximum
SUMMARY
PREVIOUS LITERATURE:
CTP derived hypoperfusion intensity ratio (HIR) is an objective and easily available imaging biomarker of tissue microperfusion which has been shown to predict infarct growth, development of malignant edema, parenchymal hemorrhagic transformation and long-term outcomes after mechanical thrombectomy for acute ischemic stroke. However, the utility of the HIR to predict early neurologic deterioration (END) and early neurologic status after mechanical thrombectomy for large vessels (LVO) versus distal and medium vessel occlusions (DMVO) has not been investigated.
KEY FINDINGS:
The optimal HIR-threshold to detect END was 0.54. HIR ≥0.54 was independently associated with a worse NIHSS score at 24h postmechanical thrombectomy and occurrence of END in LVO, but not in DMVO group.
KNOWLEDGE ADVANCEMENT:
HIR may expand its clinical utility to timely identify LVO population likely to develop severe neurologic deficits and those at risk for secondary neurologic deterioration. Further studies are needed to determine whether distinct CTP parameters should be used for outcome prediction and patient selection for endovascular treatment in DMVO.
Early neurologic status after mechanical thrombectomy (MT) as assessed by the change in NIHSS score from baseline or the absolute NIHSS score at 24 hours post-MT has been shown as a strong predictor of the 90-day functional outcome.1,2 In particular, early neurologic deterioration (END) is a feared complication after MT. Multiple clinical and procedural factors have been associated with END including age, NIHSS at presentation, the premorbid mRS score, pretreatment systolic blood pressure (SBP), the number of passes during MT, and achieved reperfusion status.3⇓-5 In addition, imaging parameters such as a low baseline ASPECTS, occlusion site, and poor collateral circulation were found to be independent predictors of END.3
The hypoperfusion intensity ratio (HIR), which can be automatically derived in real-time from CTP imaging, has been recently described as a surrogate marker of collateral status.6 A high HIR, corresponding to poor collateral status, has been shown to predict greater infarct growth,7⇓⇓-10 development of malignant edema,11 parenchymal hemorrhagic transformation,12 and worse long-term outcomes.7 However, the utility of the HIR to predict a poor NIHSS and END after MT is uncertain. In addition, there is a paucity of data about whether the HIR is useful for predicting neurologic status after MT performed on both large vessel (LVO) versus distal and medium vessel occlusions (DMVO).
In this study, we sought to determine whether a higher HIR is associated with a worse NIHSS score at 24 hours post-MT as well as END (defined as a >4-point increase in the NIHSS score between the initial assessment and 24 hours after MT) in patients with LVO and DMVO. We hypothesized that a higher HIR is associated with a higher NIHSS score at 24 hours post-MT and a worse 90-day mRS score and is an independent predictor of END.
MATERIALS AND METHODS
Study Cohort
We retrospectively analyzed consecutive adult patients with acute ischemic stroke (AIS) who underwent MT at a comprehensive stroke center between January 2020 and December 2021. Patients were included if they had LVO or DMVO amenable to MT and available CTP imaging with HIR assessment before treatment. Our investigation was approved by Corewell Health West (formerly Spectrum Health) institutional review board, and a Health Insurance Portability and Accountability Act waiver of informed consent was granted. We adhered to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines (www.strobe-statement.org).
Clinical Characteristics and Imaging Parameters
Patients’ baseline characteristics, stroke presentation details including the last known well (LKW) time, initial NIHSS score, SBP on arrival, and IV treatment with rtPA were abstracted from the medical records. Time from LKW to emergency department arrival was categorized as early (0–6 hours from LKW) versus late (>6 hours from LKW) presentation. ASPECTS and the presence of leukoaraiosis (LA) were assessed on the basis of the pretreatment NCCT. LA was defined as supratentorial WM hypoattenuation according to the STandards for ReportIng Vascular changes on nEuroimaging criteria.13 LA was graded on a 5-point scale using the scale of van Swieten et al14 and dichotomized (van Swieten scale 0–2 [absent-to-moderate] versus 3–4 [severe]). The site of the target vessel occlusion was determined on the basis of pretreatment CTA of the head and neck. LVO sites included the extracranial and/or intracranial ICA, MCA M1, and anterior cerebral artery A1 segment. We defined DMVO stroke as a stroke caused by an occlusion of the M2–4 segments of the MCA, A2–3 segments of the anterior cerebral artery, and P1–2 segments of the posterior cerebral artery (PCA). We included patients with PCA P1 occlusion consistent with the definition used in prior multicenter studies and ongoing clinical trials.15⇓⇓-18Subjects with vertebral and basilar artery occlusions were excluded from the study, given the suboptimal assessment of the posterior fossa on CTP imaging.
We graded the collateral status on the basis of the score proposed by Tan et al.19 For the study, we dichotomized the collateral status into poor (grades 0–1) versus good collaterals (grades 2–3). All perfusion studies were automatically analyzed with RAPID software (iSchemaView). We assessed relative CBF (rCBF) and time-to-maximum (Tmax) maps including rCBF <30% (ischemic core), rCBF <38%, Tmax > 10 seconds, and Tmax >6 seconds. The HIR was automatically calculated as the ratio of Tmax >10 seconds/Tmax >6 seconds. NCCT, CTA, and CTP images were reviewed independently by experienced readers blinded to the clinical data. The degree of reperfusion was assessed by the TICI score and the number of passes abstracted as documented in the procedural notes. The stroke etiology was determined using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification.20
Outcome Measures
The primary study outcome was the absolute NIHSS score recorded at 24 hours post-MT. The secondary study outcome was the occurrence of END defined as a >4-point increase in the NIHSS score between the initial assessment and 24 hours after MT. Finally, we explored whether the HIR was associated with 90-day functional outcome. For the study, we dichotomized 90-day outcome into good (mRS 0–2) versus poor (mRS 3–6). All outcome measures were assessed in the entire cohort as well as separately for the LVO and DMVO groups. All NIHSS and mRS scores were assessed by staff certified in NIHSS and mRS.
Statistics
Unless otherwise stated, continuous variables are reported as medians (25th–75th percentile). Categoric variables are reported as proportions. Between-group comparisons for continuous and ordinal variables were made with the t test, Mann-Whitney U test, 1-way ANOVA, and Kruskal-Wallis 1-way ANOVA on ranks as appropriate. Categoric variables were compared using the χ2 test or Fisher exact test as appropriate.
The optimal HIR threshold for predicting END was determined by maximizing the Youden index (sensitivity + specificity −1). To determine whether the HIR threshold was independently associated with the 24-hour post-MT NIHSS score (dependent variable), we created multivariable linear regression models for the entire cohort, as well as separately for patients with LVO and DMVO. Similarly, we created separate multivariable logistic regression models to determine whether the HIR was independently associated with END and poor 90-day outcome (dependent variables) in the entire cohort or in patients with LVO and DMVO, respectively. All models were adjusted for age, sex, baseline NIHSS score, ASPECTS, rCBF <38%, collateral status, LA severity, rtPA use, pre-MT SBP, time from LKW to presentation, TICI score, number of passes during MT, and stroke etiology. To avoid model overfitting, we sequentially removed variables (likelihood ratio) from the models at a significance level of 0.1. Collinearity diagnostics were performed (and their presence rejected) for all multivariable regression models. Model calibration was assessed by the Hosmer-Lemeshow test, and model fit was determined by examining the −2 log-likelihood statistic and its associated χ2 statistics.
Two-sided significance tests were used throughout, and unless stated otherwise, a 2-sided P < .05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics, Version 24 (IBM).
RESULTS
Study Cohort
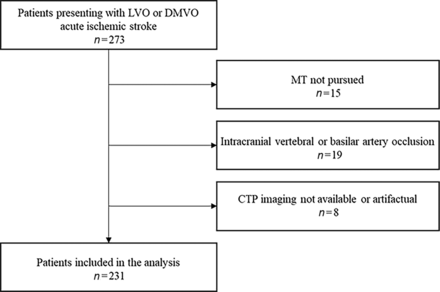
During the study period, 273 patients presented with AIS caused by an LVO or DMVO. We excluded 15 patients who did not undergo MT and 19 patients with occlusion of the intracranial vertebral or basilar artery. In addition, we excluded subjects with no or poor-quality CTP precluding HIR quantification (n = 8), leaving 231 patients for analysis (n = 125 LVO and n = 106 DMVO). Figure 1 depicts the patient flow chart. Data were complete for all variables.
Flow chart of study design and patient selection.
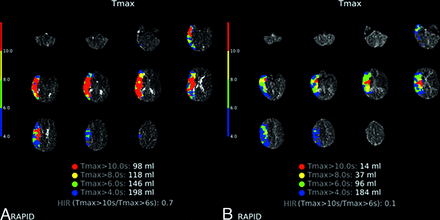
Representative imaging demonstrating HIR ≥ 0.54 (A) versus HIR < 0.54 (B) CTP profiles. A, Tmax maps illustrating HIR calculated at 0.7 by RAPID software in a 51-year-old patient who presented with an initial NIHSS score of 9, ASPECTS of 10, and right MCA M1 occlusion and underwent MT with TICI 3 reperfusion. The 24-hour post-MT NIHSS score worsened to 17, indicating the occurrence of END. B, Tmax maps illustrating HIR calculated at 0.1 by RAPID software in a 54-year-old patient who presented with an initial NIHSS score of 10, ASPECTS of 9, and right MCA M1 occlusion and underwent MT with TICI 2b reperfusion. The 24-hour post-MT NIHSS score improved to 4.
Clinical and Imaging Characteristics
Baseline clinical and imaging characteristics of the entire cohort stratified according to HIR status are presented in Table 1. Among all included patients, the optimal HIR threshold to detect END was 0.54. An HIR ≥ 0.54 was present in 48 patients (20.8%), and it was more frequently observed in the LVO compared with DMVO group (P < .001). There was no difference in age, sex, LKW to presentation time, and the use of rtPA between HIR < 0.54 versus HIR ≥ 0.54 groups (P > .05, each). Patients with HIR ≥ 0.54 had worse baseline and 24-hour post-MT NIHSS scores (P < .001, each), lower ASPECTS (P = .009), worse collaterals (P < .001), larger ischemic core, rCBF <38% (P < .001, each), mismatch (P = .048) and hypoperfusion volumes (defined either as Tmax >6 seconds and Tmax >10 seconds; P < .001, each). There was no difference in the TICI scores between HIR < 0.54 versus HIR ≥ 0.54 groups; however, in patients with HIR ≥ 0.54, significantly more passes were required to achieve reperfusion during MT compared with patients with an HIR < 0.54 (P = .030).
Patient characteristics of the entire cohort as stratified according to HIR statusa
Association of an HIR ≥ 0.54 with the NIHSS Score at 24 Hours after MT
In the entire cohort, an HIR ≥ 0.54 was independently associated with a worse NIHSS score at 24 hours post-MT after adjustment for pertinent confounders (β =0.163, P = .002; Table 2). Results were similar when we restricted the analysis to subjects with LVO. An HIR ≥ 0.54 was independently associated with a worse NIHSS score at 24 hours post-MT (β = 0.210, P = .005; Table 2). However, HIR ≥ 0.54 was not associated with 24-hour post-MT NIHSS in the DMVO group (Table 2).
Multivariable linear regression models for factors associated with NIHSS score at 24 hours post-MT in the entire cohort, patients with LVOs, and DMVOsa
Association of an HIR ≥ 0.54 with END
END occurred in 26 (11.3%) subjects. Patients with END had lower initial NIHSS scores (P = .005; Table 3), were less frequently treated with rtPA (P = .048; Table 3), and had higher pre-MT SBP (P = .010; Table 3). In addition, subjects with END required higher number of passes (P < .001; Table 3) and achieved lower TICI scores (P < .001; Table 3). Pretreatment imaging parameters such as ASPECTS, core volume, rCBF <38%, CTA collateral score, HIR, or mismatch volume did not differ between patients with versus without END (P > .05; Table 3).
Characteristics in patients with END versus without ENDa
When we analyzed the entire cohort, we found no association of HIR ≥ 0.54 with END after adjustment of pertinent clinical and imaging characteristics (Table 4). However, when we stratified our analyses, we found that an HIR ≥ 0.54 significantly increased the odds of END in subjects with LVO (OR = 5.263; 95% CI, 1.170–23.674; P = .030; Table 4) but not in the DMVO group (Table 4).
Multivariable logistic regression models for factors associated with END in the entire cohort, patients with LVOs and DMVOsa
Association of an HIR ≥ 0.54 with 90-Day Functional Outcome
We found no association of HIR ≥ 0.54 with poor 90-day functional outcome in the entire cohort (OR = 0.667; 95% CI, 0.231–1.927; P = .455). Results were similar when analyses were conducted separately for LVO (OR = 0.898; 95% CI 0.233–3.452; P = .875) and DMVO (OR = 0.446; 95% CI, 0.050–4.010; P = .471) groups.
DISCUSSION
The main finding of our study was that HIR ≥ 0.54 assessed on initial CTP imaging was independently associated with a worse NIHSS score at 24 hours post-MT and END in patients with LVO, but not DMVO.
The 24-hour NIHSS score is a strong predictor of long-term outcomes after AIS,1,2 and it is impacted by multiple clinical factors such as the premorbid mRS score, admission blood glucose level, end-stage renal disease, the use of rtPA, time from groin puncture to recanalization, and the need for general anesthesia during MT.21 In our study, in the entire cohort as well as in the LVO subgroup, an HIR ≥ 0.54 was independently associated with a worse 24-hour post-MT NIHSS score after adjustment for pertinent confounders. It was previously shown that a high HIR correlates with poor collaterals;6,7 thus, it may distinguish fast from slow progressors10,22⇓-24 and could be a useful imaging biomarker to predict the efficacy of MT.25 Our result of HIR ≥ 0.54 as predictor of the NIHSS score at 24 hours further supports the notion that the HIR may reflect a promising, easy-to-assess, imaging biomarker to aid in decision-making in patients considered for MT. Our result is in line with prior studies that showed that a high HIR is an independent factor for increased final infarction volume,7 development of intraparenchymal hematoma,12 and early malignant edema,11 which are strongly correlated with a greater neurologic deficit severity as assessed by the NIHSS score.
Notably, in contrast to patients with LVO, we did not find an independent association of an HIR ≥ 0.54 with the NIHSS score at 24 hours post-MT in the DMVO group. This finding is important because DMVOs are estimated to represent 25%–40% of all AISs,26 and it is presently not well-established which patients with DMVO are likely to benefit from MT.26 In our DMVO population, the number of passes during MT, reperfusion status, and severe LA were independently associated with the NIHSS score at 24 hours. This finding is consistent with those in previous reports indicating that procedural factors such as the number of passes and the degree of reperfusion may supersede the impact of the HIR on outcome in those with DMVO undergoing MT.27,28 As reported by Abdelrady et al,28 complete reperfusion (mTICI score 2c–3) was the strongest predictor of the favorable outcome after MT for distal M2 and M3 occlusions. In addition, as shown by Ospel et al29 in the subgroup analysis of the Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) trial, the rate of infarct progression differs on the basis of the site of the occlusion, with fast progressors being less frequent in those with more distal occlusions. Accordingly and consistent with previous reports,30 we observed overall lower HIR values in the DMVO compared with the LVO group. In addition, a high HIR was associated with an increased rate of ischemic core progression in DMVO,9,10 yet it did not correlate with clinical outcomes in this patient population. Distal occlusions may be better collateralized, and the area of tissue at risk is smaller in DMVO. Thus, smaller ischemic lesion volumes render reliable estimation of HIR in DMVO more challenging. Further studies are needed to investigate whether different HIR thresholds should be used when assessing collateral circulation in LVO versus DMVO. Likewise, it would be interesting to know if patients with DMVO have distinct CTP parameters that should be used for short- and long-term outcome prediction as well patient selection for endovascular treatment.
The incidence of END in our population (11.26%) was comparable with those reported in large MT cohorts.3,4 In agreement with previous studies,3⇓-5 we showed that older age, lower initial NIHSS score, worse collateral score, higher pretreatment SBP, and failed reperfusion status increased the odds of END in the entire cohort. Most important, we found that in the LVO subgroup, HIR ≥ 0.54 was independently associated with END. Using arterial spin-labeling MRA, Zhang et al31 showed that a high hypoperfusion volume ratio (an equivalent of HIR) is associated with END in AIS, yet the study predates the MT era. In a recent study, Faizy et al32 investigated the potential association of HIR with early neurologic improvement (ENI) and found that a favorable HIR was not associated with ENI. While the definitions for ENI and END differ, precluding direct translation between their and our results, they, nevertheless, highlighted the potential utility of using the HIR to identify patients both at increased risk of neurologic deterioration as well as a higher chance of recovery with recanalization therapy.
What is noteworthy, END comprises a variety of clinical conditions, including development of symptomatic intracranial hemorrhage and early malignant edema, both shown to correlate with a high HIR.11,12 Yet, most END causes are not clinically identifiable.33 It has been hypothesized that unexplained END may relate to collateral failure and infarct growth beyond the initial penumbral tissue. 34 In the Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3) cohort (Fig 2),35 34% of patients had infarcts larger than predicted despite successful reperfusion, and HIR in this group was significantly higher compared with patients with final infarct volume within 25 mL of prediction. Conversely, smaller-than-predicted infarcts were observed in a substantial proportion of patients with partial and unsuccessful reperfusion (21% and 43%, respectively). Indirectly, this finding may suggest that collateral status can improve or fail with time. Thus, if our data are confirmed, assessment of the viability of hypoperfused tissue by the HIR may allow identification of patients in studies seeking to determine novel strategies to prevent early deterioration. For example, selecting patients with a high HIR for cerebral blood flow augmentation such as through maintaining higher blood pressure goals may delay collateral failure and impact early clinical outcomes in this patient population. Likewise, these patients may be ideal candidates for treatment with novel neuroprotective agents aimed at improving collateral integrity and enhanced cerebral microperfusion.
A major advantage of using CTP for patient selection is that CTP is increasingly used as a part of the standard of care in AIS. HIR, which is an objective and easily available imaging biomarker of tissue microperfusion, may expand its clinical utility to timely identify the LVO population likely to develop severe neurologic deficits and those at risk of secondary neurologic deterioration. One may envision how novel artificial intelligence and machine learning tools could incorporate HIR metrics to further improve predicting neurologic outcomes and the best candidates for MT. Last, because strict clinical and imaging selection criteria for MT in patients with DMVO are not currently available, further studies are needed to determine whether distinct CTP parameters should be used for outcome prediction and patient selection for endovascular treatment in those with DMVO.
Consistent with prior observation,36 we did not find an association between HIR and 90-day functional outcome. Nevertheless, other studies reported that the HIR can be a predictor of long-term functional outcome,7,11,12,24,37 which may, in part, be explained by differences in the baseline characteristics of patients included in our study. Additional research is warranted to determine whether alternative perfusion-based collateral indices (such as the perfusion collateral index) may outperform HIR.
Strengths of the study relate to the inclusion and characterization of many patients undergoing MT for both LVO and DMVO with available CTP imaging. Furthermore, we used the 24-hour NIHSS score as the primary outcome measure, which is thought to outperform changes in the NIHSS.1 Limitations relate to the single-center design and retrospective nature of the study; thus, results should be considered hypothesis-generating. Most patients with DMVO had distal M2 and M3 occlusions, with relatively few patients having PCA P1 and P2 occlusions. Thus, it remains to be shown whether our results extend to patients with non-MCA branch occlusions. In clinical practice, PCA occlusions are not uncommon targets for MT,38 and the utility of CTP to predict outcomes in posterior circulation stroke has been reported previously.39 Last, we did not assess the causes of END, such as hemorrhagic complications or the development of early malignant edema.
CONCLUSIONS
HIR is an easily accessible metric that may expand its clinical utility to timely identify the LVO population likely to develop severe neurologic deficits and those at risk of secondary neurologic deterioration. Pending confirmation in future, prospective studies assessing the HIR may further improve predicting neurologic outcomes and finding the best candidates for MT in LVO versus DMVO AIS.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 30, 2023.
- Accepted after revision February 9, 2024.
- © 2024 by American Journal of Neuroradiology