Abstract
BACKGROUND AND PURPOSE: Fusobacterium necrophorum (F necrophorum) is an anaerobic bacteria that causes invasive head and neck infections in children. Several studies have demonstrated an increasing prevalence of F necrophorum as the causative agent in acute mastoiditis in children, with associated high rates of intracranial complications such as epidural abscess and sinus venous thrombosis, to name a few. F necrophorum requires a treatment protocol that differs from the empiric treatment that is tailored to more common pathogens (eg, group A streptococci, Streptococcus pneumonia), and hence expediting the diagnosis is important. For evaluating complicated acute mastoiditis in children, cranial CT venography remains the imaging study of choice in most medical centers due to its availability in emergency situations. Based on our clinical experience, our hypothesis is that children with F necrophorum–associated complicated acute mastoiditis can be differentiated from those with other etiologies using CT venography.
MATERIALS AND METHODS: CT venography studies of 76 children hospitalized and treated for complicated acute mastoiditis were retrospectively reviewed. Retrieved imaging data included intracranial complications (epidural abscess, sinus venous thrombosis), cranial bone-related complications, and extracranial complications (subperiosteal abscess, temporomandibular joint abscess, and soft-tissue inflammation). The cohort was divided into children with F necrophorum–related disease (study group) and those with non-F necrophorum–related disease (control group).
RESULTS: Thirty-seven children (49%) comprised the study group, and 39 children in whom the causative agents were other bacteria comprised the control group. There were significantly higher rates of complications in the study group: sinus venous thrombosis (P < .001), perisigmoid epidural abscess (P = .036), and extramastoid osteomyelitis (P < .001). Thrombosis in venous sites beyond the sigmoid sinus and jugular foramen (a pattern consistent with an otogenic variant of Lemierre syndrome) and emphysematous osteomyelitis were found only among children in the F necrophorum–related study group (32% and 22% accordingly).
CONCLUSIONS: In children with complicated acute mastoiditis, CT venography findings of emphysematous osteomyelitis and/or thrombosis in venous sites beyond the sigmoid sinus and jugular foramen (a pattern consistent with the otogenic variant of Lemierre syndrome) should lead the radiologist to suggest F necrophorum–related mastoiditis.
ABBREVIATIONS:
- AM
- acute mastoiditis
- CAM
- complicated acute mastoiditis
- CTV
- CT venography
- IJV
- internal jugular vein
- NPV
- negative predictive value
- PPV
- positive predictive value
- SVT
- sinus venous thrombosis
- TMJ
- temporomandibular joint
Acute mastoiditis (AM) in children is a serious complication of acute otitis media, with a potential to rapidly deteriorate and cause complications, both extracranial (eg, subperiosteal abscess, temporomandibular joint [TMJ] abscess) and intracranial (eg, perisigmoid epidural empyema, sinus venous thrombosis [SVT]). The clinical diagnosis of AM is based on findings of pus in the middle ear cavity associated with swelling and fluctuation over the mastoid bone, protrusion of the auricle, and fever.1 Treatment of pediatric AM has shifted from a surgically-treated disease to a mainly medically-treated disease with treatment consisting of hospitalization, middle ear drainage with myringotomy, and IV antibiotic treatment; thus, canal wall up mastoidectomy is reserved for patients with coalescent mastoiditis who do not respond to the initial therapy and/or experience complications.2⇓-4 The empiric antibiotic treatment initiated before the arrival of the results of microbiologic investigation is used to treat the most common pathogens (eg, Streptococcus pyogenes Streptococcus pneumonia)5, and in our institution, it is IV cefuroxime. In accordance with the as low as reasonably achievable concept and as was recommended by Mansour et al,6 we do not routinely image the temporal bones in children who are hospitalized and treated for AM. Imaging is reserved for children suspected of having complicated acute mastoiditis (CAM) either due to severe illness at diagnosis or nonimprovement within the initial 24–48 hours of IV antibiotocs.6,7 Imaging remains an important tool to assess the type and extent of complications for presurgical evaluation. In patients with suspected CAM, we perform cranial CT venography (CTV), which remains the imaging study of choice in most medical centers due to its availability in emergency situations.6
Several studies have demonstrated an increasing prevalence of Fusobacterium necrophorum (F necrophorum) as the causative agent in acute mastoiditis in children.8,9 F necrophorum is a non-spore-forming, obligatory anaerobic, Gram-negative rod-shaped bacteria that is responsible for a wide spectrum of infections in the head and neck, such as tonsillitis, peritonsillar abscesses, cervical lymphadenitis, sinusitis, and mastoiditis. It is also known as causative agent of Lemierre syndrome, a disease in which oropharyngeal infection is followed by septic thrombophlebitis of the internal jugular vein (IJV).10 The increased prevalence of F necrophorum–related AM in children was found to be associated with high rates of complications, including subperiosteal and epidural abscess, SVT, osteomyelitis, and the otogenic Lemierre syndrome variant (extensive septic thrombophlebitis from otogenic source of infection).8⇓-10 Due to its anaerobic nature, F necrophorum is difficult to culture and isolate, resulting in the need to use molecular diagnostic methods such as polymerase chain reaction for identification, leading to a delayed diagnosis,10 which, in our medical center, can be up to 10 days. Early diagnosis of this pathogen is crucial for administering the correct antibiotic regimen, which, in our institution, is IV ceftriaxone and metronidazole. Several studies have suggested clinical criteria for identifying children with CAM in which F necrophorum is the causative agent to facilitate early imaging and intervention. Those clinical findings include high C-reactive protein levels, high fever, substantial leukocytosis, a history of prior antibiotic treatment, and suspected subperiosteal abscess on physical examination.6,11 However, these variables are neither specific nor sensitive.
Based on our clinical experience, our hypothesis is that children with F necrophorum–associated CAM can be differentiated from those with other etiologies using CTV. To test our hypothesis, we compared the imaging characteristics of CAM in children with AM caused by F necrophorum with the imaging characteristics in children with AM caused by other bacteria.
MATERIALS AND METHODS
Patients
After study approval by the institutional ethics committee, the electronic medical records database of a university-affiliated pediatric medical center was retrospectively searched for children hospitalized and treated for acute mastoiditis from 2010 through 2021. Children who had a contrast-enhanced cranial CTV study were included; children who did not undergo a contrast-enhanced cranial CTV were excluded from the study. From the group of children with a CTV study, further exclusion criteria were the presence of cholesteatoma and an unidentified causative organism. Documented data on sex, age, and microbiologic diagnosis were retrieved from the participants’ medical charts. On the basis of the causative organism, the study participants were divided into F necrophorum–related disease (study group) and non-F necrophorum–related disease (control group).
Imaging
Per our departmental protocol for evaluating children with suspected CAM, we perform a contrast-enhanced CTV study of the skull from the mastoid tip to the vertex without a precontrast scan. Some children are placed under sedation or general anesthesia depending on their clinical status and underlying condition. Scanning is performed in the axial plane parallel to the hard palate, with a 50-second delay after a 2-mL/kg IV injection of iohexol (Omnipaque; GE Healthcare). Contrast injection is given intravenously by a power injector at a rate of 2 mL/s. Scanning parameters are 100 kV(peak), with modulated milliampere-seconds according to the patient’s size. Section thickness was 1 mm. Most studies during 2010–2013 were performed on a 64-section CT scanner (Brilliance 64; Philips Healthcare), and from 2013 to end of study period, on a 256-section multidetector CT scanner (Brilliance ICT 256). Postprocessing sequences include multiplanar reformats and bone algorithm high-resolution images.
The CTV studies were evaluated by a fellowship-trained senior pediatric neuroradiologist with Head & Neck Imaging training and 18 years of experience who was blinded to the causative agent. We documented the following imaging findings:
Intracranial Complications.
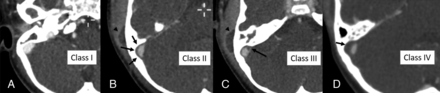
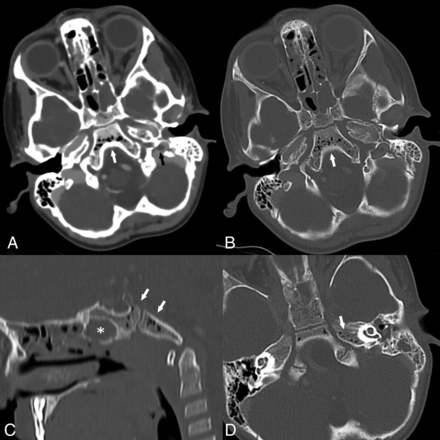
The presence of a perisigmoid abscess was evaluated by the perisinus classification as described by Horowitz et al12 and validated by Saat et al.13 According to this classification, perisinus dural lesion classes are defined as normal (class I), linear smooth halo (class II), nodular halo ≤4 mm thick (class III), and nodular halo >4 mm thick (class IV). Classes III and IV were considered positive for perisigmoid abscess (Fig 1). Epidural abscess in the middle cranial fossa was described as present or absent, and thickness was measured. Subdural empyema was described as present or absent. SVT evaluation included documentation of the presence and extent of thrombotic changes. For presence, a partial or complete filling defect in a dural venous sinus was considered positive for SVT. For extent, all venous structures affected with thrombotic changes were documented.
Perisigmoid abscess classification in 4 different cases of complicated mastoiditis: class I (A), normal dura with no thickening; class II (B), linear smooth halo of thickened dura (arrows); class III (C), focal nodular dural thickening ≤4 mm thick (arrow); class IV (D), large nodular halo >4 mm thick (arrow). Classes III and IV comprise the patients considered positive for perisigmoid abscess on imaging. Note that there is an extracranial subperiosteal abscess present in B and C (arrowhead).
Cranial Bone Complications.
Mastoid cortical erosion is a typical feature of coalescent mastoiditis and is frequently present in CAM with typical involvement of the sigmoid plate, retro auricular cortex and mastoid tegmen. We defined extramastoid bone osteomyelitis when cortical bone erosion and lytic changes were present beyond the anatomic borders of the mastoid itself. The presence of abnormal gas deposits in nonpneumatized bones, consistent with emphysematous osteomyelitis, was documented. Findings suggestive of chronic ear changes, including decreased pneumatization and increased sclerosis of mastoid bone, were also documented.
Extracranial Complications.
The presence of a subperiosteal abscess was documented, and volume was calculated on the basis of anterior-posterior, transverse, and height measurements. The presence of additional phlegmon changes was considered mild when it was retroauricular and/or adjacent to the subperiosteal abscess and extensive when larger and more extensive than the subperiosteal abscess and/or with extensive inflammatory changes to the upper neck and face. TMJ abscess was documented if present. Ipsilateral neck lymphadenopathy, ipsilateral parotid gland hyperemia, contralateral mastoid opacification, and paranasal sinus disease were also documented as present or not.
Statistics
SPSS software (IBM) was used for all statistical analyses. Categoric variables were summarized as frequency and percentage. Distribution of continuous variables was evaluated by histograms. All continuous variables were not normally distributed, and they were reported as median and interquartile range. Two-sided Fisher exact tests were used to compare categoric variables, and 2-sided Mann-Whitney tests were used to compare continuous variables. All reported P values were adjusted by the Benjamini-Hochberg procedure to control for the false discovery rate at the .05 level. The χ2 automatic interaction detector was applied to identify subgroups of patients with an increased risk for F necrophorum infection. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for findings that reached statistical significance.
RESULTS
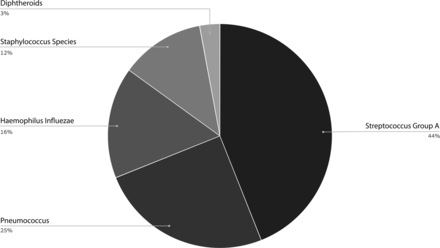
A total of 364 children were hospitalized and treated for acute mastoiditis during the study period. Ninety children met our inclusion criteria and had a CTV study due to suspected complications. From this cohort of patients, 14 children were excluded due to lack of microbiologic identification, leaving 76 children in the final combined study and control groups. Of note, 4 patients had their CTV study performed in another hospital and then were transferred to our medical center for treatment. Their CTV studies were of similar quality to our protocol, and we included them in the study. The results are summarized in Table 1. The age of the study participants ranged from 0.5 to 11.3 years (median = 2, interquartile range = 2.25), and the male/female ratio was 1.2. Thirty-seven children (49%) were diagnosed as having F necrophorum mastoiditis (the study group), while the causative agents were other bacteria in the remaining 39 children (the control group) (Fig 2). There was no significant group difference in age or sex. There was equal distribution of infection in the right and left ears in both groups.
The prevalence of bacteria species in the non-F necrophorum–related disease group.
Results
Intracranial Complications
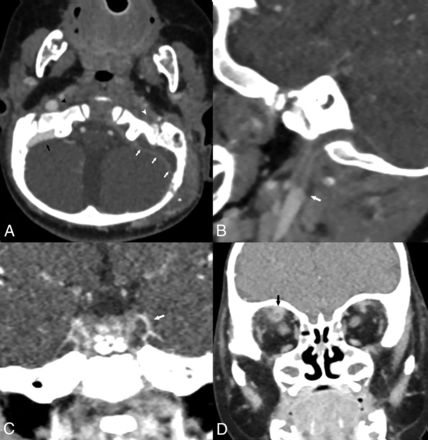
SVT was identified in 24 children in the study group (64%) and in 4 children in the control group (10%) (P < .001). Thrombosis in venous sites beyond the sigmoid sinus and jugular foramen, including the transverse sinus, IJV, cavernous sinus, and superior ophthalmic vein, was found in 12 of the 37 children (32%) in the study group and among none of the controls (Table 2 and Fig 3). Perisigmoid epidural abscess was present in 27 children in the study group (73%) and in 16 controls (41%) (P = .036). Epidural abscess in the middle cranial fossa was present in 10 children (27%) in the study group and in 4 (10%) controls (P = .199). Subdural empyema was present in 1 child (2.7%) in the study group and in 1 child (2.6%) in the control group, (P = 1).
A 9-month-old old boy with F necrophorum–related left-sided CAM causing otogenic Lemierre syndrome variant. Axial CTV image (A) at the level of the sigmoid sinus demonstrates an obstructing filling defect in the left sigmoid sinus consistent with SVT (white arrows), as apposed to normal contrast filling in the right sigmoid sinus (black arrow). No contrast is seen in the left IJV (white arrowhead) with normal contrast filling in the right IJV (black arrowhead). Also note extensive retroauricular soft-tissue phlegmon (asterisk). Sagittal-oblique MPR centered on the left jugular bulb (B) demonstrates extension of thrombus with complete obstruction of the jugular bulb and proximal IJV, with abrupt transition (arrow) where the thrombus ends. Coronal reformat of the sella region (C) demonstrates asymmetric contrast enhancement of the cavernous sinuses with a filling defect on the left (arrow), consistent with thrombosis. Coronal reformat of the orbits (D) demonstrates thrombophlebitis of the right superior ophthalmic vein with enhancement and fat-stranding along the obstructed vein (arrow).
Sites of venous thrombosis
Cranial Bone-Related Complications
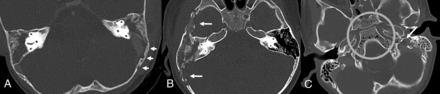
Extramastoid osteomyelitis was identified in 20 of the 37 (54%) children in the study group compared with 4 of the 39 (10%) controls (P < .001). Emphysematous osteomyelitis was diagnosed in 8 children in the study group (22%) and was not present in the control group (Fig 4).
Extramastoid osteomyelitis–related bone changes in 3 different children with F necrophorum–related CAM. A 3-year-old girl (A) with left mastoid CAM. The mastoid air cells are opacified bilaterally, but only on the left is there demineralization of the mastoid sinus plate as well as extension of focal cortical lytic changes posteriorly along the left sigmoid plate, consistent with subtle extramastoid osteomyelitis (arrows). A 2-year-old boy (B) with right-sided CAM with more extensive destructive bone changes posterior and anterior to the mastoid (arrows). A 4-year-old boy (C) with left-sided CAM has abnormal air deposits in the nonpneumatized sphenoid bone, consistent with emphysematous osteomyelitis (circle). Note also the presence of SVT in the left jugular bulb (arrow).
Extracranial Complications
A subperiosteal abscess was present in 30 children (81%) in the study group and in 30 (77%) controls, with no significant difference due to its presence (P = 1) or volume (P = .199). An abscess involving the TMJ was present in 7 children in the study group and in 1 control (P = .099) (Fig 5). The following variables showed no significant group differences: extent of inflammatory phlegmon, the presence of cervical lymphadenopathy, ipsilateral parotid gland hyperemia, opacification of the contralateral ear air cells, and sinonasal disease. Table 3 summarizes the sensitivity, specificity, PPV, and NPV for the significant variables.
An 11-month-old girl with F necrophorum–related right-sided CAM with TMJ abscess. Coronal reformat (A) and axial (B) CTV images demonstrate a large subperiosteal abscess (arrows) extending to the zygomatic arch and into the glenoid fossa of the right TMJ.
The sensitivity, specificity, PPV, and NPV for statistically significant variables
DISCUSSION
We hypothesized that children with F necrophorum–associated CAM can be differentiated from those with other etiologies using CT venography. We tested this hypothesis by comparing the imaging characteristics of CAM in children with acute mastoiditis caused by F necrophorum with the imaging characteristics in children with acute mastoiditis caused by other bacteria. Our results revealed that imaging features had very high specificity for the diagnosis of F necrophorum as the causative agent in CAM in children.
Almost one-half (49%) of the children who underwent CTV due to suspected complicated mastoiditis were infected with F necrophorum. This result supports the reported trend of increasing prevalence of F necrophorum as the causative agent in CAM in Israeli children, ranging from 8%6 to 32%14 during the past decade. These findings are not a local phenomenon and are supported by studies from other areas of the world that demonstrated an increased prevalence of F necrophorum–related otogenic infections.15,16 In addition, a national population-based study from Sweden17 reported increased F necrophorum-related head and neck and non-head and neck invasive infections.
Several studies have demonstrated a higher prevalence of intracranial complications with F necrophorum infection, with a specifically high rate of SVT.14,18⇓-20 It was, therefore, not unexpected that the most significant finding in the current study was the presence of SVT (P < .001), with high specificity (0.897) and moderate sensitivity (0.649) for F necrophorum–related CAM. SVT development in children with CAM is likely related to infection extending to the dura through infected bone or through emissary veins. The higher rate of SVT development in children with F necrophorum–associated AM reflects the invasive nature of F necrophorum infections, which cause blood vessel invasion, inflammation, and thrombosis. Virulent factors of this anaerobic bacteria include production of a lipopolysaccharide capsule, leukocidins, lipases, deoxyribonuclease, hemolysins, hemagglutinins, neutrophil-cytotoxic factors, and the ability to aggregate platelets and produce proteolytic enzymes that enhance invasion.10 In addition, host factors such as prior viral infection or immune system immaturity may also play a role.8,10 The prevalence of SVT in our study group was 62%, which is much higher than the rates reported in other studies: Gelbart et al14 reported a prevalence of 21% in 19 children with F necrophorum and Ulanovski et al19 reported a prevalence of 36.6% in 41 patients with F necrophorum. It is unclear though if the increased rate of SVT in this study is related to changes in F necrophorum virulence or in clinical parameters in the studied population. This question will need to be further addressed in future prospective studies.
Our results showed the importance of documenting the location and extent of thrombosis. SVT involving venous structures beyond the sigmoid sinus and jugular bulb was found to be present in 12 (32%) children in the study group and in none of the controls. These findings differ from those reported by Coudert et al,16 who did not observe any significant difference in the extent of SVT between FN mastoiditis and mastoiditis related to other bacteria. However, those authors defined the extent of SVT in relation to sigmoid sinus obstruction as being partial, complete, or extended (involving the sigmoid sinus and the jugular vein), and it is unclear whether their report includes a distinction between the jugular bulb and the IJV or if they documented thrombosis in distant venous structures. The pattern of extensive thrombophlebitis that we encountered in this study is consistent with the otogenic Lemierre syndrome variant (Fig 3), which was also described in 4 of 14 pediatric cases of F necrophorum–related complicated mastoiditis in a study by Le Monnier et al,15 and in 7 of 12 cases reviewed by Stergiopoulou and Walsh.8 Lemierre syndrome is a serious complication of head and neck F necrophorum infection characterized by thrombophlebitis of the jugular venous system, bacteremia, and possible septic embolism, leading to necrotizing pneumonia or involvement of multiple organs or joints.10 The otogenic Lemierre syndrome variant in our study was found to be unique to F necrophorum and not encountered in our control group, thus establishing it as an important pattern to be recognized by the radiologist to suggest F necrophorum infection.
Another significant finding with clinical importance was the presence of extramastoid bone erosions, reflecting osteomyelitis beyond the coalescent mastoiditis, which was present in 20 study group children (54%) compared with 4 controls (4%) (P = .00036). Additionally, a pattern of gas bubbles in a nonpneumatized mastoid and/or extramastoid bone was found in 8 study group children (22%) compared with none of the controls. The finding of extramastoid osteomyelitis was also described in 5 of the 12 cases (42%) reviewed by Stergiopoulou and Walsh,8 in 4 of the 19 cases (21%) described by Gelbart et al,14 and in 3 of the 7 cases (43%) described by Yarden-Bilavsky et al.18 The authors of the latter study described 1 case of extensive osteolysis with “small gas bubbles in the soft tissue surrounding the mastoid cells” depicted in a CT study, but it is unclear from their description if gas bubbles were present in the bone itself. Gas in bone as a manifestation of F necrophorum osteomyelitis has been described in skeletal bones as a complication of F necrophorum bacteremia.21 Osteomyelitis related to gas-forming organisms in an adult population was referred to as emphysematous osteomyelitis, and the unique pattern of gas deposits in the bone, such as the one we encountered in this pediatric study, was termed a “pumice stone” pattern due to its resemblance to the surface appearance of pumice stone.22 Those authors also noted a lack of cortical destruction in most cases of pumice stone pattern, as had also been seen among the children presenting with this pattern of bone destruction in our study (Fig 6). This pattern is most discernable in nonpneumatized bones (eg, clivus prepneumatization), and it helps to compare the affected bone with the contralateral side for comparison of the pneumatization pattern. The pumice stone pattern of osteomyelitis is considered rare,22 but our findings suggest that it is an important pathognomonic pattern to recognize in F necrophorum–related CAM in the pediatric population.
A 4-year-old boy with left-sided F necrophorum–related CAM complicated with a pumice bone pattern extramastoid osteomyelitis. The hallmark of this pattern is abnormal air deposits in a nonpneumatized bone without extensive cortical destruction, as seen in this child. Axial CTV image in a soft-tissue window (A) and in the bone algorithm (B) demonstrates abnormal air deposits in the nonpneumatized clivus (white arrows). A sagittal reformat (C) shows that both sphenoidal and basilar parts of the clivus are involved (arrows), distinguished from the sphenoid sinus that has mucosal thickening (asterisk). Similar changes are seen in the left petrous apex (arrow) (D) compared with the normal bone in the right petrous apex. Note also the presence of SVT in the left jugular bulb (back arrow) (A).
Abscess involving the TMJ is an important complication of acute mastoiditis, which may lead to ankyloses if untreated.23,24 We found more cases of TMJ abscess in the study group (7 children) compared with the control group (1 child). Although this difference did not reach a level of significance (P = .099), we believe it is of clinical importance, in agreement with Burgess et al,24 who also reported F necrophorum as the most common pathogen in their case series of 9 children with complicated mastoiditis-related TMJ septic arthritis.
The most common complication we encountered was extracranial subperiosteal abscess, which was present in 30 study group children (81%) and 30 controls (77%). However, there was no significant difference in the presence (P = 1) or volume (P = .199) by which to differentiate F necrophorum from other bacteria. Our findings differ from a study by Coudert et al,16 which reported significantly higher volumes of subperiosteal abscess in F necrophorum acute mastoiditis. The second most common finding in our study was intracranial perisigmoid epidural abscess, with a significantly higher prevalence in the F necrophorum group (P = .036), but with moderate sensitivity and specificity (0.73 and 0.59, respectively).
Our findings suggest that the radiologist plays an essential role in suggesting the likelihood of F necrophorum–related infection. This is of clinical importance because the clinical course of F necrophorum–related CAM in children differs significantly from that of other pathogens related to CAM. Ulanovski et al19 reported that children with F necrophorum–related mastoiditis had a statistically significantly more complex postoperative course and a significantly longer IV treatment period, which averaged 22 days for F necrophorum–related disease versus 7 days for other pathogen-related disease (P = .0001). Expediting the diagnosis based on the imaging characteristics will allow an early change of IV antibiotic protocol, guide a more aggressive surgical approach, and facilitate parental counseling in preparation for a more protracted healing process, including the need for extended IV antibiotics treatment, which requires, in many cases, a peripherally inserted central catheter placement.
In this study both otogenic Lemierre syndrome variant–related findings and pumice stone pattern emphysematous osteomyelitis were pathognomonic to F necrophorum infection. The presence of SVT (PPV = 0.857) or extramastoid osteomyelitis (PPV = 0.833) or a combination of both (PPV = 1) is highly suggestive of F necrophorum. The presence of a perisigmoid epidural abscess is not highly specific for F necrophorum (PPV = 0.63), but in combination with SVT (PPV = 0.826) or extramastoid osteomyelitis (PPV = 0.889), the positive predictive value increases. The sensitivity of imaging findings is not as high as the specificity; hence, the NPV is not high enough to allow exclusion of F necrophorum–related disease, especially due to the increasing prevalence of F necrophorum as the causative agent in pediatric CAM.
Our study has a few limitations. First, its retrospective nature precludes precision verification of the retrieved clinical data and complete standardization of imaging technique, because patients were scanned on >1 CT scanner, including 4 children who were referred to our tertiary medical center with imaging studies performed elsewhere. Of note, all CTV studies were found to be of adequate technique to allow diagnosis. Second, the study design did not include an additional reader, precluding assessment of interobserver variability. Third, statistical evaluation was limited by the small number of subjects with clinically important findings, such as the presence of thrombosis in the superior ophthalmic vein, suggesting that a prospective multicenter study will be warranted to further validate our findings. Fourth, this study did not include an MR imaging correlation, which may enhance our understanding of disease extent in pediatric CAM and will need to be addressed in future studies.
CONCLUSIONS
In children with CAM, CTV findings of emphysematous osteomyelitis and/or thrombosis in venous sites beyond the sigmoid sinus and jugular foramen (a pattern consistent with the otogenic Lemierre syndrome variant) should lead the radiologist to suggest F necrophorum–related mastoiditis.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received October 22, 2023.
- Accepted after revision February 5, 2024.
- © 2024 by American Journal of Neuroradiology