Abstract
BACKGROUND AND PURPOSE: T2-FLAIR mismatch is a highly specific imaging biomarker of IDH-mutant diffuse astrocytoma in adults. It has however also been described in MYB/MYBL1–altered low grade tumors. Our aim was to assess the diagnostic power of the T2-FLAIR mismatch in IDH-mutant astrocytoma and MYB/MYBL1–altered low-grade tumors in children and correlate this mismatch with histology.
MATERIALS AND METHODS: We evaluated MR imaging examinations of all pediatric patients, performed at the Princess Máxima Center for Pediatric Oncology and the University Medical Center Utrecht between January 2012 and January 2023, with the histomolecular diagnosis of IDH-mutant astrocytoma, diffuse astrocytoma MYB/MYBL1–altered, or angiocentric glioma, and the presence of T2-FLAIR mismatch was assessed. Histologically, the presence of microcysts in the tumor (a phenomenon suggested to be correlated with T2-FLAIR mismatch in IDH-mutant astrocytomas in adults) was evaluated.
RESULTS: Nineteen pediatric patients were diagnosed with either IDH-mutant astrocytoma (n = 8) or MYB/MYBL1–altered tumor (n = 11: diffuse astrocytoma, MYB- or MYBL1-altered n = 8; or angiocentric glioma n = 3). T2-FLAIR mismatch was present in 11 patients, 3 (38%) in the IDH-mutant group and 8 (73%) in the MYB/MYBL1 group. No correlation was found between T2-FLAIR mismatch and the presence of microcysts or an enlarged intercellular space in either IDH-mutant astrocytoma (P = .38 and P = .56, respectively) or MYB/MYBL1–altered tumors (P = .36 and P = .90, respectively).
CONCLUSIONS: In our pediatric population, T2-FLAIR mismatch was more often found in MYB/MYBL1–altered tumors than in IDH-mutant astrocytomas. In contrast to what has been reported for IDH-mutant astrocytomas in adults, no correlation was found with microcystic changes in the tumor tissue. This finding challenges the hypothesis that such microcystic changes and/or enlarged intercellular spaces in the tissue of these tumors are an important part of explaining the occurrence of the T2-FLAIR mismatch.
ABBREVIATIONS:
- NGS
- next-generation sequencing
- WHO
- World Health Organization
Pediatric-type low-grade astrocytoma with MYB or MYBL1 alteration is a newly defined CNS tumor type listed in the 5th edition of the World Health Organization (WHO) CNS tumor classification.1 It is considered a CNS WHO grade 1 tumor, despite the infiltrative growth into the CNS parenchyma, which is the defining feature of grade 2 gliomas.2,3 The WHO CNS tumor classification describes 2 types of grade 2 tumors with a MYB or MYBL1 alteration: 1) diffuse astrocytoma, MYB or MYBL1–altered,1,4 and 2) angiocentric glioma with a MYB-QKI fusion.5 These types of tumors show radiologic and histologic differences but have molecular overlap with MYB or MYBL1 alterations as tumor drivers and close clustering in DNA methylation profiling studies.2,6
A few years ago, Kalelioglu et al7 published 2 cases of pediatric-type low-grade astrocytomas with MYB/MYBL1 alteration with their MR imaging characteristics. They also mentioned T2-FLAIR mismatch as a possible imaging marker. This mismatch was defined as a tumor with a homogeneous high T2-weighted MR signal intensity that showed a decreased signal in the central part and a hyperintense rim on FLAIR. In adults, T2-FLAIR mismatch has emerged as a highly specific imaging biomarker for IDH-mutant astrocytoma, with a reported specificity of 100%.8 How often T2-FLAIR mismatch is seen in MYB/MYBL1–altered low-grade tumors and in IDH-mutant astrocytomas in children has yet to be elucidated. This information is highly relevant because the MYB/MYBL1–altered tumors are designated CNS WHO grade 1 and have a better prognosis than IDH-mutant diffuse astrocytomas, which can progress to a CNS WHO grade 4 tumor.6
Therefore, we performed a retrospective study to assess the presence of T2-FLAIR mismatch in pediatric patients with a MYB/MYBL1–altered tumor or IDH-mutant astrocytoma. A second aim was to test the hypothesis that microcystic change and/or an enlarged intercellular space as seen by histopathologic analysis in adult-type IDH-mutant astrocytomas with T2-FLAIR mismatch underlies the mismatch in these pediatric tumors as well.9
MATERIALS AND METHODS
Relevant clinical, histopathologic, molecular, and imaging data of all consecutive pediatric patients from the Princess Máxima Center for Pediatric Oncology and the University Medical Center Utrecht with the histomolecular diagnosis of diffuse astrocytoma, MYB/MYBL1–altered, angiocentric glioma, or IDH-mutant astrocytoma were collected from January 2012 until January 2023.
Imaging
All patients underwent a dedicated brain tumor imaging protocol, including T2-FLAIR, T2 TSE, and T1-weighted imaging before and after contrast administration and DWI. MR imaging studies were performed at field strengths of 1.5T and 3T. The imaging protocol slightly changed during the period of inclusion. However, T2-FLAIR and T2 TSE were always available. Good interrater agreement (κ = 0.75) for the T2-FLAIR mismatch was found using a cohort with different field strengths and acquisition protocols.10
Characteristics and results of the cases with astrocytoma, IDH-mutant, CNS WHO grades 2–4
The imaging features assessed were T1 and T2 signal intensity of the tumor, location, existence of only solid or also cystic parts, diffuse infiltrative versus more expansile with sharp borders (on T2 FLAIR and T2), diffusion restriction (visual assessment of the ADC map with restriction being areas of low signal compared with normal brain tissue), contrast enhancement of the solid part of the tumor, and T2-FLAIR mismatch.
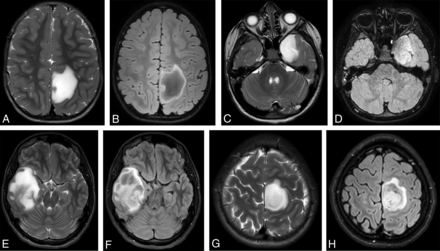
Because not all our cases showed the T2-FLAIR mismatch in the whole tumor, we distinguished between the T2-FLAIR mismatch sign (Fig 1A, -B, -E, and -F) and T2-FLAIR mismatch area (Fig 1C, -D, -G, and -H) using the following definitions:
T2-FLAIR mismatch sign and area in MYB/MYBL1–altered diffuse astrocytoma and IDH-mutant glioma, T2- and T2-FLAIR-weighted sequences. A and B, MYB/MYBL1–altered diffuse astrocytoma left parietal lobe, mismatch sign. C and D, MYB/MYBL–altered diffuse astrocytoma left temporal lobe, mismatch area. E and F, IDH-mutant astrocytoma right temporal lobe, mismatch sign. G and H, IDH-mutant astrocytoma left frontoparietal lobe, mismatch area.
T2-FLAIR Mismatch Sign.
T2-FLAIR mismatch in the central solid part of the whole tumor shows a high signal on T2 and a lower signal on FLAIR with a thin hyperintense rim on the FLAIR image.
No necrotic or cystic parts in the tumor are visible on the MR images.
T2-FLAIR Mismatch Area.
Homogeneous hyperintense T2-weighted signal only in the solid part of the tumor
FLAIR hypointense signal, with a surrounding hyperintense rim around the same (solid) area but without a hyperintense rim around the nonsolid parts of the tumor.
Two neuroradiologists (with 2 and 8 years of experience in pediatric neuro-oncology) evaluated these features independently, blinded to the molecular diagnosis.
Pathobiology
All patients underwent surgery for biopsy or (partial) resection. Three board-certified neuropathologists evaluated all patients’ tumors histologically, immunohistochemically, and molecularly, and all tumors on review were diagnosed according to the CNS WHO 2021 classification.
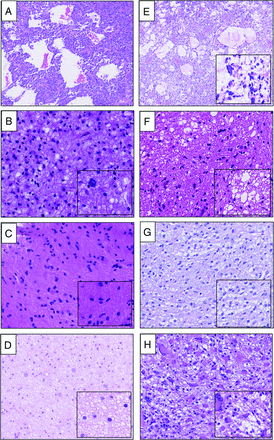
The molecular analysis included DNA methylation profiling, next-generation sequencing (NGS) panel mutation analysis, and/or whole transcriptome sequencing. Histologic re-evaluation included assessment of the presence/absence of atypia, mitotic activity, and necrosis, with special attention to the presence/absence of microcysts and enlarged intercellular spaces described by Yamashita et al.9 The presence of microcystic change was determined to be positive if true microcysts could be detected at overview (with the 0.5× objective), and the presence of an enlarged intercellular space was determined to be positive if true microcysts could be detected with the 10× objective (Fig 2B, -D).
A–D, Low-grade tumors, MYB/MYBL1–altered. A, T2-FLAIR mismatch and microcysts in an angiocentric glioma. B, T2-FLAIR mismatch and an enlarged intercellular space (EIS). C, T2-FLAIR mismatch without microcyst or EIS. D, No T2-FLAIR mismatch with EIS. B–D, All from diffuse astrocytomas, MYB/MYBL1–altered. E–H, Diffuse astrocytoma, IDH-mutant. E, T2-FLAIR mismatch and microcysts in grade 3 astrocytoma. F, No T2-FLAIR mismatch with microcysts in grade 2 astrocytoma. G, No T2-FLAIR mismatch and no microcyst or EIS in grade 2 astrocytoma. H, No T2-FLAIR mismatch with EIS in grade 2 astrocytoma. Magnification, 50–100×; inset, 200–400×.
Statistical Analyses
The interrater reliability for the T2-FLAIR mismatch was determined using the Cohen κ coefficient. The presence of the T2-FLAIR mismatch (sign and area) between the MYB/MYBL1 group and the IDH-mutant group was statistically analyzed using the Fisher exact test for independent proportions.
For further analyses, consensus on the presence of T2-FLAIR mismatch was reached in all cases, again blinded to the molecular diagnosis.
The differences in T2-FLAIR mismatch between the IDH-mutant and the MYB/MYBL1–altered group were statistically analyzed using the Fisher exact test for independent proportions. Correlations between T2-FLAIR mismatch on the one hand and the presence of histologic microcysts or an enlarged intercellular space on the other were tested using the Kendall rank correlation coefficient.
RESULTS
Nineteen pediatric patients were identified with either an IDH-mutant astrocytoma (2 grade 2, five grade 3, and 1 grade 4) or a MYB/MYBL1–altered tumor (8 diffuse astrocytomas, MYB/MYBL1–altered, and 3 angiocentric gliomas, all grade 1). One of our patients in the IDH-mutant astrocytoma group underwent surgery shortly after 18 years of age. Characteristics and the imaging/pathobiologic results of the 2 groups are outlined in Tables 1 and 2. Additional imaging characteristics are outlined in the Online Supplemental Data.
Characteristics and results of the cases with diffuse astrocytoma MYB/MYBL1–altered or angiocentric glioma, CNS WHO grade 1
Imaging
In 15/19 cases, the 2 neuroradiologic reviews were concordant (κ = 0.58). Consensus was reached for discrepant findings in these 4 cases.
T2-FLAIR mismatch was present in 10 patients (Fig 1), 8 in the MYB/MYBL1 group (73%), and 2 in the IDH-mutant group (25%) (P = .07). Of the 8 cases in the MYB/MYBL1 group with T2-FLAIR mismatch (sign and area), 6 were diffuse astrocytomas, MYB or MYBL1–altered, and 2 were angiocentric gliomas. The angiocentric gliomas showed a T2-FLAIR mismatch area in the solid parts; additional cystic parts were also visible on MR imaging. None of the angiocentric gliomas had a T2-FLAIR mismatch sign. No MYB/MYBL1–altered diffuse astrocytoma or angiocentric glioma showed restricted diffusion or enhancement after gadolinium. In the IDH-mutant diffuse astrocytomas, there was restricted diffusion in 2 (25%) cases, both in WHO grade 2 tumors. Sharp borders have been noted in 9 of 11 diffuse astrocytomas, MYB/MYBL1–altered, and only 3 of 8 in the IDH-mutant cases.
Correlation of Pathology and Imaging
Histopathologically, all MYB- or MYBL1–altered tumors showed diffuse growth of relatively monomorphic glial cells with ovoid or spindled nuclei within a fibrillar matrix. There was no frank atypia, mitotic activity, florid microvascular proliferation, or necrosis; all were CNS WHO grade 1. Only 2 cases showed histologically clear-cut microcystic changes, and both were found to have T2-FLAIR mismatch (18%). Three cases with T2-FLAIR mismatch showed an enlarged intercellular space of limited size (27%), while 3 other cases with T2-FLAIR mismatch did not show either microcystic changes or an enlarged intercellular space in the tissue that was available for histopathologic analysis (27%). Of the 3 cases without T2-FLAIR mismatch, 2 cases had enlarged intercellular spaces (Fig 2A–D).
The IDH-mutant astrocytomas showed more variable histology. Most cases were in line with CNS WHO grade 2 (moderate atypia, low mitotic activity, and no necrosis), but 1 case was regarded as grade 4 because of increased mitotic activity, pronounced nuclear pleomorphism, palisading tumor necrosis, and extensive florid microvascular proliferation. Two cases showed T2-FLAIR mismatch, both with microcystic changes (25%). Of the 6 cases that were negative for T2-FLAIR mismatch, 4 cases also had microcysts, and 3 cases showed enlarged intercellular spaces comparable with, or even more pronounced than those in the MYB/MYBL1–altered tumors (Fig 2E–H).
Microcysts were significantly more often observed in IDH-mutant astrocytomas than in MYB/MYBL–altered tumors (P = .02). However, there was no correlation of a T2-FLAIR mismatch and the presence of microcysts in the IDH-mutant astrocytoma group (τ = 0.33, P = .38). Furthermore, no significant correlation was found between T2-FLAIR mismatch and the presence of an enlarged intercellular space in IDH-mutant astrocytoma (τ = 0.22, P = .56). Also, in MYB/MYBL1–altered tumors, no significant correlation was found between T2-FLAIR mismatch and the presence of microcysts or an enlarged intercellular space (τ = 0.29, P = .36 and τ = –0.04, P = .90, respectively).
DISCUSSION
T2-FLAIR mismatch is a noninvasive biomarker that can identify patients with IDH-mutant lower-grade astrocytomas with a positive predictive value up to 100% in the adult population.10,11 This biomarker has not been not tested thoroughly in the pediatric population, in which the occurrence and specificity for IDH-mutant astrocytoma is still being determined. Kalelioglu et al,7 for example, reported a T2-FLAIR mismatch in 1 of their 2 patients with a MYB/MYBL1–altered diffuse astrocytoma.
In this study, we have shown both the presence and histologic correlation of T2-FLAIR mismatch in children, not only in IDH-mutant astrocytomas but also in MYB/MYBL1–altered tumors (either diffuse astrocytoma, MYB/MYBL1–altered, or angiocentric glioma). The T2-FLAIR mismatch was present in 25% of the IDH-mutant astrocytomas and in 73% of MYB/MYBL1 tumors. Acknowledging the possibility of a MYB/MYBL1–altered tumor is essential because there is an important difference in prognosis and treatment compared with IDH-mutant diffuse astrocytoma. Chiang et al2 showed that 10-year progression-free survival and overall survival rates were 90% and 95% in patients (median age, 5 years; range, 0–26 years) with MYB/MYBL1–altered tumors. In contrast, adults with lower-grade IDH-mutant astrocytoma, 1p/19q-noncodeleted, show worse outcomes with a median survival period of 6.3 years.12 In children, the clinical impact of IDH1 mutation is less clear. These tumors may have the same biology as in the adult malignancy but are identified earlier in life. At least, they probably behave less indolently than other pediatric low-grade diffuse astrocytomas, and patients should be more closely followed.13
In our cohort, only 25% showed a T2-FLAIR mismatch in the IDH-mutant, 1p/19q-noncodeleted cases, which is lower than that in the adult population, which ranges around ≥50% in the IDH-mutant, 1p/19q-noncodeleted cases. Furthermore, 73% of MYB/MYBL1 tumors showed a T2-FLAIR mismatch. Therefore, in the case of a pediatric CNS tumor with a T2-FLAIR mismatch with additional MR imaging characteristics compatible with a more low-grade appearance (no enhancement, no diffusion restriction), neuroradiologists should also consider a MYB/MYBL1–altered tumor in their differential diagnosis. This suggestion especially holds true for pediatric cases with the T2-FLAIR mismatch sign, which we almost exclusively observed in our MYB/MYBL1–altered diffuse astrocytoma cases. In contrast, in our (small) case series, the T2-FLAIR mismatch area was relatively frequently observed in angiocentric glioma. When a T2-FLAIR mismatch is observed, clinicians may be more hesitant to follow a watch-and-wait strategy because of the IDH-mutant astrocytoma in the differential diagnosis. The clinical consequence would be to obtain tissue to be confident of the histopathologic diagnosis. The finding of the T2-FLAIR mismatch in the more indolent MYB/MYBL1–altered tumors might change the radiologic differential diagnosis.
Additionally, the location of the tumor can be influential. We had only 1 MYB/MYBL1–altered infratentorial diffuse astrocytoma; all other cases were supratentorial, like all our IDH-mutant tumor cases. Therefore, in case of an infratentorial T2-FLAIR mismatch area, the presence of a MYB/MYBL1–altered angiocentric glioma should be especially considered.
Diffusion restriction or enhancement, commonly seen in high-grade brain tumors, was also not noted in our MYB/MYBL1–altered tumors cases.
There is still debate on the tissue characteristics that may underlie the T2-FLAIR mismatch phenomenon (sign or area). The newest insights are that T2-FLAIR mismatch in IDH-mutant astrocytoma is due to microcystic changes and/or enlarged intercellular spaces within the tumor that contain enough fluid volume to cause suppression on FLAIR images.9 A histopathologic review of our cases showed microcystic changes and/or an enlarged intercellular space in 64% of MYB/MYBL1–altered tumors and 88% of IDH-mutant astrocytomas. However, the T2-FLAIR mismatch sign was observed just as often in MYB/MYBL1–altered tumors and IDH-mutant astrocytomas without microcystic changes or enlarged intercellular spaces. In our case series, a clear-cut correlation was absent. One explanation might be the sampling effect, because in some of our cases, only needle biopsy material was available for histologic analysis. Moreover, we did observe some intratumoral histologic heterogeneity for microcystic change and/or enlarged intercellular spaces in cases in which larger tumor fragments were available for evaluation. Another contributing factor might be the decreased statistical power of our analyses due to the small sample size.
In our cohort study in children, T2-FLAIR mismatch was observed in some of the IDH-mutant astrocytomas but even more frequently in MYB/MYBL1–altered tumors, both in angiocentric gliomas and diffuse astrocytomas, MYB/MYBL1–altered. Of note, T2-FLAIR mismatch has now also been reported in an H3K27M-mutant (and IDH wild-type) diffuse midline glioma in the brainstem of an adult.14 A diffuse midline glioma is a tumor type that histologically typically does not show prominent microcystic change or an enlarged intercellular space.4 In contrast, microcysts are frequently present in pilocytic astrocytomas.15 However, T2-FLAIR mismatch has not been reported in these patients in relevant series,16 and we did not observe it in our own center either. Again, this challenges the hypothesis that such microcystic changes are an important part of the explanation for the occurrence of the T2-FLAIR mismatch sign.
Finally, a recent study revealed that the inversion time used for the FLAIR sequence and field strength impacts the diagnostic accuracy of T2-FLAIR mismatch for low-grade astrocytoma imaging.17 In our cases, the assessment of the T2-FLAIR mismatch will not be influenced by the field strength because all imaging was performed on 3T MR imaging systems (Phillips) at our institute, nor by different inversion times because all FLAIR imaging was performed with a 3D FLAIR sequence with nearly the same parameters.
Our study has some limitations. First, the number of cases is relatively limited. Although it seems clear that T2-FLAIR mismatch is clearly present in MYB/MYBL1–altered tumors, more evidence is needed to show the diagnostic value of T2-FLAIR mismatch for this type of tumor in the pediatric population. Second, the patient selection was retrospective with the identification of all patients with a MYB/MYBL1–altered, angiocentric glioma or IDH-mutant astrocytoma. From these data, the false-positive rate of T2-FLAIR mismatch can, therefore, not be determined. Also, some patients with MYB/MYBL1–altered, angiocentric glioma or IDH-mutant astrocytoma may have been missed due to less extensive molecular tumor characterization in the past.
CONCLUSIONS
In our study, T2-FLAIR mismatch was found not only in grade 1–4 IDH-mutant astrocytomas but also (and even more frequently) in grade 1 MYB/MYBL1–altered tumors in children. Therefore, the radiologic differential diagnosis should include a grade 1 MYB/MYBL1–altered tumor when evaluating the MR imaging of pediatric CNS tumors. Furthermore, our study challenges the hypothesis that microcystic changes and/or enlarged intercellular spaces in the tissue of these tumors are essential for causing T2-FLAIR mismatch.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received June 23, 2023.
- Accepted after revision January 23, 2024.
- © 2024 by American Journal of Neuroradiology