SUMMARY:
Spontaneous intracranial hypotension is characterized by symptoms of low intracranial CSF volume due to various mechanisms of CSF leakage. One such mechanism is a CSF-venous fistula, treatable with transvenous embolization resulting in substantial radiographic and clinical improvement. However, the exact mechanisms underlying these improvements, including the potential involvement of the glymphatic system, remain unclear. To noninvasively assess glymphatic clearance in spontaneous intracranial hypotension, we used an advanced MR imaging technique called the DTI along the perivascular spaces in 3 patients with CSF-venous fistula before and after embolization. All 3 patients with spontaneous intracranial hypotension initially had low glymphatic flow, which improved postembolization. Two patients with symptomatic improvement exhibited a more substantial increase in glymphatic flow compared with a patient with minimal improvement. These findings suggest a possible link between cerebral glymphatics in spontaneous intracranial hypotension pathophysiology and symptomatic improvement, warranting larger studies to explore the role of the glymphatic system in spontaneous intracranial hypotension.
ABBREVIATIONS:
- CSFVF
- CSF-venous fistula
- DTI-ALPS
- DTI analysis along the perivascular space
- FA
- fractional anisotropy
- SIH
- spontaneous intracranial hypotension
Spontaneous intracranial hypotension (SIH) is an orthostatic headache syndrome caused by several possible mechanisms of CSF leakage with resultant low intracranial CSF volume.1⇓-3 CSF-venous fistula (CSFVF) has recently been identified as an important cause of SIH. Likewise, treatment of CSFVF with transvenous embolization has emerged as a robust treatment strategy with substantial radiographic and clinical improvement.4⇓-6 However, while symptomatic improvement may presumably be attributed to improved CSF dynamics and normalized intracranial CSF volume, questions remain regarding the impact of SIH on cerebral blood flow and metabolism, including cerebral glymphatic clearance. Broadly, the glymphatic system is a cerebral metabolic waste-removal/drainage system involving CSF diffusion along the perivascular space.7 Given that SIH often results from abnormal CSF drainage via a CSFVF, it is conceivable that SIH may also be related to impaired glymphatic outflow. Prior studies have noninvasively quantified cerebral glymphatic clearance in several CNS diseases by leveraging the DTI analysis along the perivascular space (DTI-ALPS), an advanced MR imaging technique.8⇓-10 However, no studies have evaluated glymphatic clearance in patients with SIH or investigated how it might change following transvenous embolization of the culprit CSFVF. Here, we report 3 patients with SIH with impaired cerebral glymphatic flow, which improved following transvenous embolization of the CSFVF.
MATERIALS AND METHODS
From the 7 patients with CSFVF who were treated with transvenous embolization at our tertiary care institution at the University of Rochester Medical Center, 3 patients had sufficient pre-/postembolization MR imaging required to evaluate their cerebral glymphatic outflow using the DTI-ALPS technique. Abbreviated details regarding the DWI sequence processing are described below. Additionally, clinical and radiographic parameters were collected, including the pre-/postembolization Bern Score.11 At 3-month follow-up, postembolization brain MR imaging was acquired along with clinical assessment of symptomatic change.
Glymphatic DWI Analysis
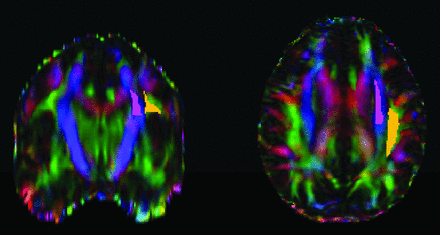
The DTI-ALPS technique measures the diffusivity along the perivascular space of the medullary veins, the primary drainage pathway of the glymphatic system, and has been previously validated and robustly described in prior studies.8⇓-10 The DWI data were corrected for eddy current–induced distortion using EDDY_CORRECT in FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki//eddy). Susceptibility-induced distortion was corrected using INVERSION, consisting of inverse contrast normalization of T1WI data and diffeomorphic coregistration using symmetric normalization in Advanced Normalization Tools (ANTs; http://stnava.github.io/ANTs/).12 Diffusivity maps along the x-axis (Dxx), y-axis (Dyy), and z-axis (Dzz) were computed in addition to fractional anisotropy (FA), mean diffusivity, axial diffusivity, and radial diffusivity using DIPY (https://docs.dipy.org/stable/index.html). FA maps were coregistered to the FA map template FMRIB58 atlas using ANTs. The FA registration matrix was used to warp all other DTI maps to standard space. Modified JHU ICBM (https://www.researchgate.net/figure/Johns-Hopkins-University-ICBM-DTI-81-White-Matter-Labeled-Atlas-regions-of-interest-on_fig1_349013706) WM labels were used for the projection (superior corona radiata) and association fibers (superior longitudinal fasciculus) in the periventricular area. An example of an atlas-based ROI determination can be seen in Fig 1. The ALPS index, which is used as a measure of glymphatic clearance, was then computed using these labels, defined by
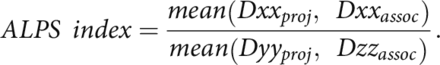
Sample of ROI determination using the atlas-based ALPS index. The projection (superior and posterior corona radiata, magenta ROI) and association (superior longitudinal fasciculus, yellow ROI) fibers were defined by the labels of the ICBM DTI-81 Atlas.
For purposes of reference and scale, a literature normal ALPS index is approximately 1.29, and a literature minimum value can be as low as 0.93.10
RESULTS
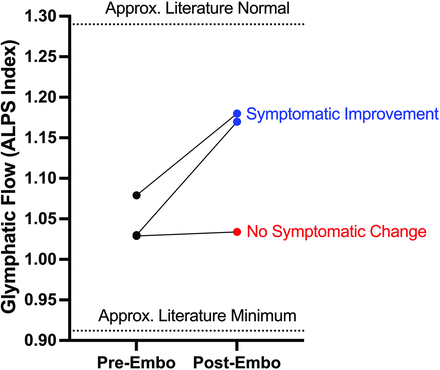
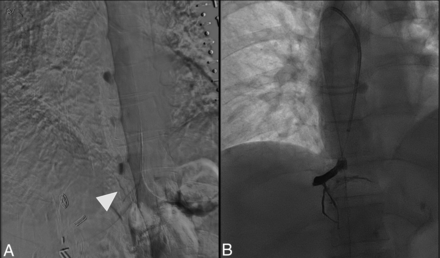
Three patients with SIH due to CSFVF were identified with sufficient MR imaging for analysis before and after transvenous embolization. Two patients were women, and one was a man; the average age was 65 years. The average preoperative Bern Score on MR imaging was 6.7. Each of the 3 patients had a lower than (literature) normal ALPS index suggestive of impaired glymphatic flow at baseline (Fig 2). All 3 patients had right-sided thoracic CSFVFs on digital subtraction myelography, which were subsequently embolized with Onyx (Medtronic) (example can be seen in Fig 3). Two patients (patients 1 and 2) had substantial clinical improvement of their symptoms (defined as >75% improvement) after embolization and a decrease in the Bern Score on imaging (Online Supplemental Data). Likewise, patients 1 and 2 had improvement in their glymphatic clearance after embolization on 3-month follow-up (Online Supplemental Data, Fig 2). Patient 3 had only slight improvement in glymphatic clearance (ALPS index, 1.029–1.034), with mild improvement in the Bern Score and no notable change in his clinical symptoms (Online Supplemental Data).
Change in cerebral glymphatic clearance in patients with SIH after transvenous embolization of a CSFVF. Embo indicates embolization; Approx., approximate.
A, Patient 1 is a representative example of CSFVF (white arrowhead) on digital subtraction myelography located on the right at the level of T10. B, Onyx cast within the foraminal vein, intercostal vein, and adjacent venous plexus following transvenous embolization.
DISCUSSION
It is entirely unknown whether glymphatic clearance is altered in patients with SIH due to CSFVF and if/how it may contribute to symptomatology or pathophysiology. In this brief report, all 3 patients demonstrated impaired baseline glymphatic clearance (ALPS Index, 1.029–1.078) compared with a literature normal reference value (ALPS Index, ∼1.29).10 If a minimum literature ALPS Index value is considered to be approximately 0.93, this suggests that patients with CSFVF may have <50% of baseline glymphatic outflow compared with healthy patients. Additionally, in all 3 patients, CSFVF embolization at least partially recovered glymphatic function, suggesting that the observed low ALPS values might be attributed to the CSFVF.
Although the mechanism underlying the presented observation is unknown, it has been previously shown that abnormal intracranial pressure alters CSF clearance pathways, suggesting a possible connection between intracranial pressure homeostasis and cerebral glymphatic flow.13 Likewise, it is also possible that glymphatic function may be pressure-dependent, in which a decrease in outflow might parallel the decreased CSF volume. Additionally, we hypothesize that general brain sagging due to low intracranial CSF volume may structurally distort the cerebral venoglymphatic system and could result in the observed impaired flow. Furthermore, venous sinus engorgement, a common neuroimaging fining in SIH, could possibly compress and compromise the adjacent cerebral perivascular glymphatics. Last, low CSF intracranial volume could conceivably alter the homeostasis of the cerebral interstitial space, which may compromise cerebral interstitial compliance and the glymphatic system.14
While purely theoretical, one potential implication of this study is that it could explain some CSFVF symptoms that are not as well-explained by gross structural abnormalities such as brain sag. In general, it is accepted that brain sag with resultant traction of pain-sensitive fibers or trigeminal nerve ganglia is the possible reason for headaches. However, other common SIH symptoms and complications including drowsiness, brain fog, and frontotemporal dementia are not as well-explained by brain sag. Given that impaired glymphatic flow has been implicated as the final common pathway for dementia, it is possible that this may be a mechanism for some of the harder-to-localize symptoms of SIH due to CSFVF.7
In addition to possibly implicating the glymphatic system in SIH due to CSFVF, the DTI-ALPS method could be used for diagnostic purposes. It is feasible that the extent of impaired glymphatic flow could function as a quantitative neuroimaging marker for patients with CSFVF and may theoretically be combined with the Bern Score to aid in the diagnosis. However, impaired glymphatic flow is nonspecific to SIH and can be seen in other conditions, which may limit this possibility.9,10 Regardless, given the diagnostic challenge of SIH and CSFVF, a low ALPS index obtained noninvasively from a brain MR imaging could serve as an additional data point for further work-up, including a diagnostic myelogram. Similarly, while not previously investigated, the ALPS index could also assist in the diagnosis and management in patients with a low Bern Score, which could be explored in future studies.
While the DTI-ALPS method is noninvasive and can be completed retrospectively, additional techniques have been described to image the glymphatic system. For example, following IV or intrathecal administration of gadolinium-based contrast, MR cisternography can be used to track CSF transport and measure glymphatic clearance along the paravascular routes.15 Meningeal lymphatic vessels can also be directly imaged via FLAIR MR imaging following contrast administration, when the rate of meningeal lymphatic efflux reflects the rate of paravascular/glymphatic outflow.15 Additional advanced MR imaging techniques include intravoxel incoherent motion and chemical exchange saturation transfer to measure cerebral interstitial fluid transport.15 These methods, among others, could also be used to study the glymphatic system in patients with SIH.
While the obvious limitation of this study is the modest sample size of 3 patients and absence of age-matched controls, in all instances, glymphatic flow increased following transvenous embolization. Most interesting, we also observed that in the 2 patients with symptomatic improvement, the postembolization glymphatic flow increase was notably higher than the minimal increase in patient 3, who demonstrated a mild radiographic improvement and no appreciable symptomatic improvement (Online Supplemental Data, Fig 2). This finding could suggest that the extent of glymphatic clearance rescue may at least partially reflect/explain the substantial radiographic and clinical improvement that is often observed with transvenous embolization.4⇓-6 Alternatively, normalized intracranial volume after CSFVF embolization may structurally restore the venoglymphatic system, resulting in at least partially normalized glymphatic flow. Nonetheless, a change in glymphatic clearance could conceivably function as a quantitative marker of treatment success based on our modest n = 3. An additional limitation is that we only included patients with SIH with an identified CSFVF. It is possible that SIH due to other mechanisms (ventral tear and so forth) may not have impaired glymphatic clearance as suggested here. Future studies researching the glymphatic system in SIH should investigate CSF leaks of different etiologies and with appropriate controls.
CONCLUSIONS
The glymphatic flow in patients with SIH due to a CSFVF can be quantitatively measured with the DTI-ALPS method. This small series suggests that patients with SIH have impaired glymphatic clearance of unclear etiology and mechanism and that transvenous embolization could restore glymphatic flow. Although this series does not function as proof, this report does open a new line of inquiry in SIH. Additional studies are required to validate the presented findings and to clarify the role that the glymphatic system might play in SIH.
Footnotes
Funding was provided by the Department of Imaging Sciences, University of Rochester Medical Center via the Fischer Fund Grant.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
references
- Received January 8, 2024.
- Accepted after revision February 6, 2024.
- © 2024 by American Journal of Neuroradiology