Abstract
BACKGROUND AND PURPOSE: Fluorine 18-fluoro-L-dopa ([18F]-FDOPA) was approved by the FDA in 2019 and reimbursed by the Centers for Medicare & Medicaid Services in 2022 for use with PET to visualize dopaminergic nerve terminals in the striatum for evaluation of parkinsonism. We sought to determine the optimal image acquisition time for [18F]-FDOPA PET by evaluating rater-estimated FDOPA positivity and image quality across 4 time points.
MATERIALS AND METHODS: Brain PET/CT was acquired 90 minutes following injection of 185 megabecquerel (5 mCi) of [18F]-FDOPA. PET was acquired in list mode for 20 minutes, and data were replayed to represent 15-, 10-, and 5-minute acquisitions. By means of MIMneuro, PET/MR imaging or PET/CT was independently graded for FDOPA positivity and image quality by 2 readers, blinded to the clinical report and diagnosis. Expert neuroradiologist clinical reads were used as the criterion standard.
RESULTS: Twenty patients were included, average age 65.6 years, 55% women. Image-quality ratings decreased with shorter acquisition times for both readers (reader 1, ρ = 0.23, P = .044; reader 2, ρ = 0.24, P = .036), but there was no association between abnormality confidence scores and acquisition time (reader 1, ρ = –0.13, P = .250; reader 2, ρ = –0.19, P = .100). There was a high degree of consistency in intra- and interrater agreement and agreement with the expert reads when using acquisition times of ≥10 minutes (maximal confidence score consistency [ρ = 0.92] and interrater agreement [κ = 0.90] were observed at 15 minutes), while image quality was consistently rated as low and FDOPA positivity ratings were inconsistent when using a 5-minute acquisition time.
CONCLUSIONS: Our study suggests that image-quality ratings were stable after 15 minutes and that between-subject abnormality detection rates were highly consistent between the 2 readers when acquired for at least 10 and up to 20 minutes but were inconsistent at 5 minutes. Shorter [18F]-FDOPA PET acquisition times may help maximize patient comfort while increasing throughput in the clinical setting.
ABBREVIATIONS:
- AADC
- aromatic amino acid decarboxylase
- AC
- attenuation-corrected
- DaT
- dopamine transporter
- PD
- Parksinson disease
- PS
- parkinsonian syndromes
Parkinsonian syndromes (PS) are a heterogeneous group of disorders that present with motor symptoms that are mostly ascribed to idiopathic Parkinson disease (PD). This group includes PD as well as atypical PS such as multiple system atrophy, progressive supranuclear palsy, dementia with Lewy bodies, and corticobasal degeneration. PS are distinguished from other causes of parkinsonism (essential tremor, drug-induced parkinsonism, and vascular parkinsonism) by the presence of nigrostriatal degeneration. This distinction is crucial to initiate the appropriate therapy and prevent the possible harmful effects of unsupported levodopa therapy when the symptoms mimic PD but are not neurodegenerative. Functional imaging with dopamine ligands can aid with this distinction by targeting either the presynaptic or postsynaptic dopaminergic system.1
Several radiotracers are currently used for presynaptic dopaminergic molecular imaging, with the most common being radiopharmaceuticals N-3-fluoropropyl-2-(R)- carboxymethoxy-3-(R)-(4-[iodine 123] iodophenyl) nortropane (123I FP-CIT, dopamine transporter scan [DaTscan]) and 3,4-dihydroxy-6-[fluorine 18 fluoro-L-dopa ([18F]-FDOPA).2 [18F]-FDOPA is a radiolabeled analog of L-DOPA used to image metabolic abnormalities of presynaptic DaTs and L-type amino acid transporters and may be used for the assessment of PS.3 Tracer accumulation in the striatum corresponds to the level of radiotracer uptake into the presynaptic nerve terminal, dopa-decarboxylase (aromatic amino acid decarboxylase [AADC]) activity, and vesicular storage in the presynaptic dopaminergic neurons.4,5 Studies have demonstrated that the uptake of [18F]-FDOPA in the basal ganglia is reduced in PD,6 thereby facilitating the differentiation between PS and non-neurodegenerative causes of parkinsonism. Characteristic findings in patients with PD include asymmetric reduction of [18F]-FDOPA uptake in the striatum, with greater reductions typically noted contralateral to the clinically more-affected side.7,8 In addition, radiopharmaceutical binding is lowest in the posterior putamen compared with the anterior putamen and caudate nucleus, which is referred to as the rostrocaudal gradient of FDOPA uptake.9,10 Reductions in [18F]-FDOPA uptake have consistently been shown to best correlate with disease severity and clinical bradykinesia scores, while the correlation with tremor, rigidity, and postural disturbance is less significant.11⇓⇓⇓-15
[18F]-FDOPA has been FDA-approved to visualize dopaminergic nerve terminals in the striatum for the evaluation of adult patients with suspected PS since 2019 and received Centers for Medicare & Medicaid Services payment approval in late 2022.16,17 Our current institutional protocol for [18F]-FDOPA brain PET is in accordance with the FDA prescribing guidelines18 and includes list mode acquisition for 20 minutes, though acquisition times for PET vary depending on scanner sensitivity and administered dose. Notably, the highest uptake of the radiotracer in the striatum is approximately 90 minutes postinjection of [18F]-FDOPA.19,20 Using dynamic acquisitions, we noted anecdotally that the clarity of the images might imply that a shorter acquisition time could be sufficient for the assessment of nigrostriatal dysfunction in the clinical setting. There are several potential benefits to a shorter acquisition time. First, patients with parkinsonism often have trouble lying still in the scanner for long periods owing to tremors or other movement disorders or cognitive impairments, so a shorter PET scan duration may improve patient comfort and reduce motion artifacts. Additionally, shorter acquisition times could allow increased throughput of patients, enabling greater access and allowing more patients the opportunity to be evaluated by this emerging technique. Therefore, we sought to determine the optimal image acquisition time for [18F]-FDOPA brain PET by evaluating reader confidence and image quality across 4 acquisition periods.
MATERIALS AND METHODS
We started our clinical FDOPA PET imaging service in November 2022, and the first 20 patients imaged at our institution were selected for this study. Patients were instructed to withhold PD medications, if applicable, for 12 hours before the PET scan, and followed a low-protein diet beginning in the evening before the examination, with nothing by mouth except for water for 4 hours before the examination. All subjects were pretreated with carbidopa, 150 mg, approximately 1 hour before IV injection of 185-megabecquerel [5-mCi] [18F]-FDOPA. Brain PET/CT was acquired on a Discovery 710HD scanner (GE Healthcare) 90 minutes following injection in list mode for 20 minutes and reconstructed according to the standard institutional protocol (Table 1). Following each 20-minute acquisition, data were reconstructed to represent 15-, 10-, and 5-minute acquisition times.
[18F]-FDOPA brain PET/CT protocola
Following Northwell institutional review board approval, the attenuation-corrected (AC) PET data across 4 time points were anonymized, and all indicators of acquisition time were removed, as were the CT and separately acquired brain MR imaging studies for each subject. These anonymized studies were then analyzed by 2 readers, blinded to the clinical report and final diagnosis. These 2 readers were the same for every patient and consisted of senior residents in diagnostic radiology with clinical experience reading DaTscans. Both readers participated in multiple training sessions for FDOPA PET interpretation using MIMNeuro software (Version 7.3.2; MIM Software) before study commencement. By means of MIMneuro, findings of [18F]-FDOPA PET/MR imaging or PET/CT (if MR imaging was unavailable) were independently graded for reader confidence on the likely FDOPA positivity of an image using a 5-point scale (0 = definitely normal, 1 = probably normal, 2 = equivocal, 3 = probably abnormal, 4 = definitely abnormal). For analyses of inter- and intrarater agreement and when comparing scores with the expert rater, confidence scores were dichotomized to indicate the presence/absence of abnormal findings using a cutoff of ≥3 (probably abnormal). Image quality was graded on a 3-point scale (1 = poor quality, 2 = acceptable, 3 = excellent); poor-quality images were those rated as 1 in this scale.
Expert clinical reads by a board-certified fellowship-trained neuroradiologist with an MD/PhD and 10 years of brain PET/MR imaging clinical and research experience were used as the criterion standard.
Statistical Analysis
Sample characteristics were described using means (SDs) or percentages if appropriate and stratified by neuroradiology expert reads (Tables 2 and 3). Nonparametric trend tests were used to determine whether demographic characteristics, image quality, and abnormality confidence scores varied by FDOPA abnormality. Next, intraindividual consistency was measured using the Spearman rank-order correlation within individuals to determine the extent to which FDOPA images were rated consistently in terms of confidence of abnormal findings and image quality across scanning parameters. Because the Spearman ρ may be biased in cases in which rankings remain stable though average scores differ, the Cohen κ was also used to examine the presence/absence of abnormal imaging findings for all rater times and when comparing raters and the neuroradiologist’s expert reads. To visually differentiate these measures, we used a different cell color (red, green, and blue) to differentiate the test type; then darker hues of each color were used to indicate stronger associations (Table 4). Two-tailed t tests were used to examine the statistical significance of the Spearman ρ and Cohen κ in all tests. Because of the large number of comparisons and the small sample size made up of relatively unique patients, statistical significance in the final analyses was reported in 2 ways. Bold italicized typeface was used to report statistically significant results that passed the false discovery rate (0.05). Supplemental analyses were completed to examine image-quality ratings using χ2 tests to estimate the impact of acquisition time on the raters’ ratings of image quality. However, while we did attempt to account for image-quality rankings when considering positivity scores, due to the small sample size, these efforts were unsuccessful in most cases and these analyses are not presented. Analyses were completed by using STATA 17/MP (StataCorp).
Demographic characteristics for subjects (n = 20) stratified by neuroradiologist expert read results at the standard 20-minute acquisition time
Reader confidence and image quality scores stratified by neuroradiologist expert read results at the standard 20-minute acquisition timea
Measures of image-rating agreement and consistency across the 2 raters and the expert neuroradiologist read using different acquisition timesa
RESULTS
Demographic and clinical characteristics for n = 20 subjects included in the study are depicted in Tables 2 and 3. The patients had an average age of 65.6 years, and 55% were women. No differences were evident in demographic characteristics between groups, but the confidence and quality scores differed between groups across all ratings. In general, the scans rated by the neuroradiologist’s expert read as having abnormal findings had higher confidence scores and lower image-quality ratings than those rated as having normal findings.
Per the neuroradiologist’s expert read, 10 studies (50%) had abnormal findings as evaluated at the standard 20-minute acquisition time (Fig 1). There was no association between reader confidence and acquisition time (reader 1, ρ = –0.13, P = .250; reader 2, ρ = –0.19, P = .100). However, image-quality ratings were lower among images with shorter acquisition times for both reviewers (reader 1, ρ = 0.23, P = .044; reader 2, ρ = 0.24, P = .036) (Figs 2 and 3).
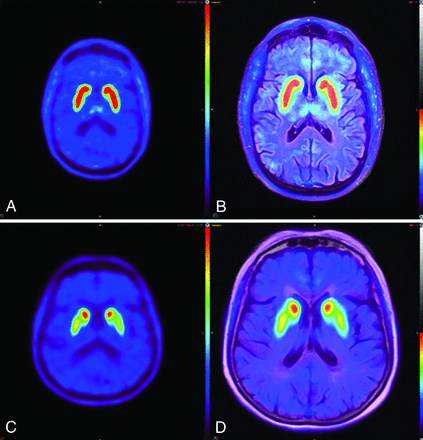
[18F]-FDOPA brain PET and fused [18F]-FDOPA brain PET/MR imaging at a standard 20-minute acquisition time. The healthy subject (A and B) demonstrates the characteristic “comma sign” uptake in the striatum. The subject with abnormal findings (C and D) demonstrates marked loss of uptake in the bilateral striatum, particularly involving the bilateral putamina and left > right caudate nuclei, corresponding to more extensive right-sided clinical deficits.
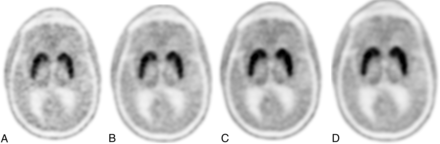
Normal findings on [18F]-FDOPA AC PET at 5-minute (A), 10-minute (B), 15-minute (C), and 20-minute (D) acquisition times. Note that image-quality ratings were stable following 15 minutes, and between-subject abnormality detection rates were highly consistent between the 2 readers when using 10-, 15-, or 20-minute acquisition times but were inconsistent at 5 minutes.
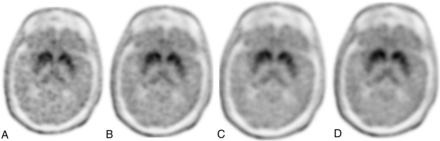
Abnormal findings on [18F]-FDOPA AC PET at 5-minute (A), 10-minute (B), 15-minute (C), and 20-minute (D) acquisition times. Note that image-quality ratings were stable following 15 minutes, and between-subject abnormality detection rates were highly consistent between the 2 readers when using 10-, 15-, or 20-minute acquisition times but were inconsistent at 5 minutes.
Analysis of interrater reliability (Table 4) revealed that an abnormal finding on an image was rated similarly between readers at 20-, 15-, and 10-minute acquisition times. However, the interrater confidence scores were inconsistent across raters and between the raters and the expert neuroradiologist read at 5 minutes. In contrast, ratings were relatively consistent both in terms of rank order and abnormality agreement when using image-acquisition times of 10–15 minutes. Of interest, the maximal association in confidence scores between raters occurred at 15 minutes (ρ = 0.92), while the maximal κ when comparing raters also occurred at 15 minutes (κ = 0.90).
Not shown as main results are examinations of image-quality ratings made by the 2 readers (Online Supplemental Data), which showed relatively less consistent results. For example, the raters consistently scored image quality lower when acquisition times were 5 minutes (ρ = 0.68, P = .001), compared with 10-minute (ρ = 0.36, P = .124), 15-minute (ρ = 0.43, P = .058), or 20-minute (ρ = 0.29, P = .209) acquisition times. Raters agreed that image quality was poor in most scans when using a 5-minute acquisition time (Online Supplemental Data).
DISCUSSION
Functional imaging with dopamine ligands targets the dopaminergic system and is currently used to aid with the distinction of PD from other conditions that mimic the disease. Following the FDA approval of [18F]-FDOPA PET in 2019, which was obtained on the basis of a clinical trial of 56 patients with suspected PS,16,21 we introduced [18F]-FDOPA PET into practice and incorporated this study into our routine clinical workflow for PD assessment, because it is currently reimbursed by the Centers for Medicare & Medicaid Services in the United States.17 The present study sought to examine the influence of imaging-parameter selection and quality on abnormality ratings and found that the optimal acquisition times for [18F]-FDOPA PET for clinical purposes was approximately 10–15 minutes.
The European Association of Nuclear Medicine and the Society of Nuclear Medicine and Molecular Imaging have developed joint clinical practice guidelines that address clinical and technical aspects of dopaminergic imaging for patients with PS, to assist radiologists in recommending, performing, interpreting, and reporting the results of dopaminergic imaging in patients with PS.22 Per these guidelines, the diagnostic importance of presynaptic dopaminergic imaging (ie, [18F]-FDOPA PET; DaT SPECT) is the following: 1) to support the differential diagnosis between essential tremor and neurodegenerative PS;23⇓⇓-26 2) to help distinguish dementia with Lewy bodies and other dementias (in particular, Alzheimer disease);27⇓-29 3) to support the differential diagnosis between PS due to presynaptic degenerative dopamine deficiency and other forms of PS (eg, drug-induced, psychogenic, or vascular parkinsonism);30⇓-32 and 4) to detect early presynaptic PS.33,34
The goal of [18F]-FDOPA PET visual assessment is to qualitatively analyze uptake in the striatum (putamen and caudate nucleus) by setting the maximum color scale value to the maximal tracer value in the striatum. Moreover, [18F]-FDOPA not only allows qualitative interpretation, but there are established quantitative parameters that can be calculated to objectively quantify the degree of striatal neuronal loss.35⇓⇓-38 Semiquantitative analysis may be performed in the clinical setting, typically by calculating the striato-occipital ratio, which has been shown to correlate with clinical disability ratings.35 Quantitative analysis of dynamic time-activity curves may also be used to determine multiple aspects of [18F]-FDOPA influx constants (Ki maps).36 For example, Dhawan et al38 demonstrated a graphic approach to compare the striatal-to-occipital ratio and influx constant in [18F]-FDOPA PET studies, highlighting a similar accuracy using a short 10-minute scan at 95 minutes post-radiopharmaceutical injection. Similarly, in our study, visual image-quality ratings were stable following 15 minutes, and between-subject abnormality detection rates were highly consistent between 2 readers when using 10-, 15-, or 20-minute acquisition times but were inconsistent at 5 minutes. While acquisition times for PET vary depending on the scanner sensitivity and the administered dose, these results suggest that neuroradiologists may consistently and reliably interpret [18F]-FDOPA PET at acquisition times shorter than the traditionally used 20 minutes.
Several studies have found that [18F]-FDOPA PET and the DaTscan are highly accurate and diagnose PD with similar levels of sensitivity and specificity.2,19,39 There are several major advantages that favor the use of [18F]-FDOPA PET over DaT SPECT.2,39⇓⇓-42 These include improved image resolution (∼5mm for PET versus ∼13-14 mm for SPECT), shorter imaging sessions, lower radiation burden, similar-to-reduced cost, and no risk of potential iodine-induced thyroid-related side effects and therefore no need for the administration of the Lugol solution before the examination to fully saturate the thyroid to protect it from radiation exposure.41 Furthermore, the ability to fuse [18F]-FDOPA PET with either CT or MR structural imaging and the introduction of simultaneous PET/MR imaging scanners for the assessment of movement disorders provides the ability to correlate with concurrent anatomic abnormalities (eg, lacunar infarcts in the striatum; enlarged perivascular spaces), which may provide added value and help confirm or exclude the existence of concomitant pathologies.43
There are several limitations to [18F]-FDOPA PET dopaminergic imaging. For example, Wallert et al40 suggested that [18F]-FDOPA PET may be less sensitive than DaTscans in patients with clinically uncertain PS in the clinical setting. This suggestion may relate to the challenges of interpretation in routine practice outside the research setting. A meta-analysis has also suggested that the decrease in activity in the striatum of patients with PD is consistently smaller in studies assessing [18F]-FDOPA PET compared with DaT SPECT.10 Furthermore, in early disease, there is a relative increase in [18F]-FDOPA uptake compared with vesicle monoamine transporter 2 imaging, with the radiotracer carbon 11 [11C] dihydrotetrabenazine likely corresponding to compensatory upregulation of AADC activity in early-stage PD.5 Finally, limitations specific to our study include its relatively small sample size, lack of quantitative analyses (we focused on visual interpretation only in an attempt to closely mimic routine clinical practice), and absence of long-term follow-up of study subjects for clinical monitoring of disease progression.
CONCLUSIONS
[18F]-FDOPA PET is a promising emerging technique for the assessment of parkinsonism. Despite several minor limitations, our study suggests that image-quality ratings were stable at 10–15 minutes, and that between-subject abnormality detection rates were highly consistent between 2 readers when using 10-, 15-, or 20-minute acquisition times but were inconsistent at 5 minutes. As this novel dopaminergic imaging technique translates into clinical practice, shorter PET acquisition times may help maximize patient comfort while increasing throughput in the clinical setting.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 7, 2023.
- Accepted after revision January 25, 2024.
- © 2024 by American Journal of Neuroradiology