Abstract
BACKGROUND AND PURPOSE: Acute mountain sickness is a series of brain-centered symptoms that occur when rapidly ascending to high altitude. Predicting acute mountain sickness before high-altitude exposure is crucial for protecting susceptible individuals. The present study aimed to evaluate the feasibility of predicting acute mountain sickness after high-altitude exposure by using multimodal brain MR imaging features measured at sea level.
MATERIALS AND METHODS: We recruited 45 healthy sea-level residents who flew to the Qinghai-Tibet Plateau (3650 m). We conducted T1-weighted structural MR imaging, resting-state fMRI, and arterial spin-labeling perfusion MR imaging both at sea level and high altitude. Acute mountain sickness was diagnosed for 5 days using Lake Louise Scoring. Logistic regression with Least Absolute Shrinkage and Selection Operator logistic regression was performed for predicting acute mountain sickness using sea-level MR imaging features. We also validated the predictors by using MR images obtained at high altitude.
RESULTS: The incidence rate of acute mountain sickness was 80.0%. The model achieved an area under the receiver operating characteristic curve of 86.4% (sensitivity = 77.8%, specificity = 100.0%, and P < .001) in predicting acute mountain sickness At sea level, valid predictors included fractional amplitude of low-frequency fluctuations (fALFF) and degree centrality from resting-state fMRI, mainly distributed in the somatomotor network. We further learned that the acute mountain sickness group had lower levels of fALFF in the somatomotor network at high altitude, associated with smaller changes in CSF volume and higher Lake Louise Scoring, specifically relating to fatigue and clinical function.
CONCLUSIONS: Our study found that the somatomotor network function detected by sea-level resting-state fMRI was a crucial predictor for acute mountain sickness and further validated its pathophysiologic impact at high altitude. These findings show promise for pre-exposure prediction, particularly for individuals in need of rapid ascent, and they offer insight into the potential mechanism of acute mountain sickness.
ABBREVIATIONS:
- AMS
- acute mountain sickness
- ASL
- arterial spin-labeling
- AUC
- area under the curve
- DC
- degree centrality
- fALFF
- fractional amplitude of low-frequency fluctuations
- LASSO-LR
- Least Absolute Shrinkage and Selection Operator logistic regression
- LLS
- Lake Louise Score
- rs-fMRI
- resting-state fMRI
- ROC
- receiver operating characteristic
- SMN
- somatomotor network
- SpO2
- saturation of pulse oxygen
Approximately 25%–90% of sea-level residents who travel to high altitude will have acute mountain sickness (AMS), depending on the altitude, the speed of ascent, and individual susceptibility.1 AMS is a series of symptoms including headache, dizziness, and malaise. It can even lead to incapacitation or life-threatening conditions such as high-altitude cerebral edema and pulmonary edema.2
It was recommended that high-altitude travelers have medical consulting regarding AMS before exposure.3 Predicting AMS before exposure is also essential for protecting individuals from greater health risks. Because rapid ascent is a risk factor of AMS,1 individuals requiring rapid ascent to high-altitude areas might particularly need the prediction, such as those undertaking urgent high-altitude missions like disaster relief, medical rescue, and some scientific investigations. Currently, 2 main factors show promise in predicting AMS: the history of AMS and migraine4 and the adaptation performance in moderate hypoxia, such as the cardiopulmonary function in artificial hypoxic environments4⇓⇓-7 and adaptation extents at moderate altitudes.8⇓⇓⇓-12 However, the prediction performance was inconsistent and debated, because studies have reported conflicting results using the same predictors.13⇓⇓-16 Furthermore, most individuals have limited high-altitude experience, making prediction based on history less feasible. The artificial hypoxic environments, often requiring specialized labs, are also debated for not adequately replicating the hypobaric conditions of real high altitude.17
The inconsistent performance in predicting AMS may be due to an incomplete understanding of the mechanisms.18 Among the hypotheses, the brain plays a key role in both human and animal research.19,20 The physiologic adaptation after high-altitude exposure is brain-centered and crucial for the onset of AMS, which is hypothesized to involve multiple neural processes such as the perception and processing of the sensory information from a high-altitude environment21,22 and the activation of the autonomic neural system.23,24 The swelling of gray and white matter, the morphologic changes of the cortex, the restricted outflow of CSF, and the increased CBF can increase intracranial pressure and thus cause AMS,25,26 while the contributions of different components toward AMS are still debated.27 Those hypotheses involve multiple aspects of the brain, including function, structure, and perfusion. However currently, multimodal MR imaging studies for AMS scanned at both sea level and high altitude are still rare and have not involved integrated pre-exposure prediction.28,29 The feasibility of using multimodal MR imaging to investigate and predict AMS requires further verification.
In this study, we arranged for 45 participants to fly from sea level to the Qinghai-Tibet Plateau. They underwent 3 modalities of MR imaging twice (before and after high-altitude exposure) and were diagnosed as having AMS or non-AMS. On the basis of the previous studies, we hypothesized that the AMS group would have distinct cerebral features at sea level, detectable by multimodal MR imaging, and that we could further investigate its pathophysiologic impact using MR imaging after exposure.
MATERIALS AND METHODS
Study Design
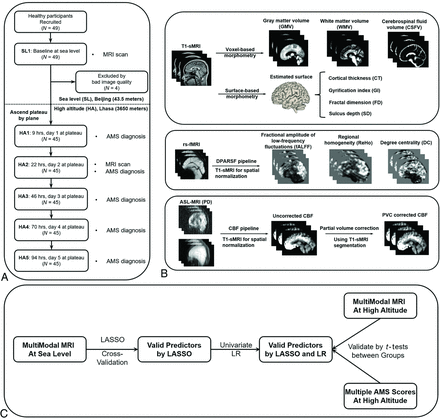
This study was conducted in both sites of the Chinese People’s Liberation Army General Hospital (Beijing, at sea level) and The General Hospital of Tibet Military Region (Lhasa, 3650 m above sea level). In Beijing as a baseline, participants were scanned by 3 modalities: T1-weighted imaging, resting-state fMRI (rs-fMRI), and arterial spin-labeling (ASL) perfusion MR imaging. Then, participants traveled to Lhasa via commercial flights within 2 days. In Lhasa, participants underwent the same MR imaging protocol at 22 hours and were diagnosed as either having AMS or not having AMS during the first 5 days at the following time points: 9, 22, 46, 70, and 94 hours. The flow chart of the study design is shown in Fig 1A. Demographic and physiologic features were recorded at baseline for prediction.
The study design and the analysis pipelines for multimodal MR images. A, The study design. Participants were recruited and underwent MR imaging at sea level, ascended to the high altitude by plane, were scanned by MR imaging at 22 hours, and evaluated for AMS 5 times at high altitude. B, Multimodal MR imaging preprocessing. Different preprocessing pipelines produced 11 features from 3 modalities of MR imaging, and these features were further summarized by the mean value in ROIs. C, Feature selection. The sea-level MR imaging features for prediction of AMS were selected by LASSO-LR and re-verified by univariate logistic regression. Sea-level predictors were subsequently verified using high-altitude MR imaging and AMS scores (LLS) collected at high altitude. DPARSF indicates Data Processing Assistant for Resting-State fMRI; LR, logistic regression; PD, proton density–weighted images; PVC, partial volume correction.
Participants
A total of 49 participants were recruited through community advertising. Four participants were excluded due to strong head-motion artifacts on MR imaging at baseline, identified after post hoc checking. Two rs-fMRIs from the 45 participants at high altitude were excluded due to poor image quality, but sea-level data from these 2 participants were still valid and included for prediction.
The recruitment criteria were as follows: 1) 20–40 years of age; 2) no history of severe head trauma, chronic headache, chronic sleep disorders, other neuropathy, or psychosis; 3) nonsmokers and not abusing alcohol; 4) not taking any prescription or nonprescription medications at the time of the study; 5) no chronic heart or lung disease, diabetes, hypertension, or other basic diseases; 6) right-handed; 7) originally from and currently residing at sea level and not having traveled to an altitude above 1500 m in the past year; and 8) no carotid or intracranial vascular lesions detected by MRA.
AMS Diagnosis
The AMS diagnosis is based on the latest Lake Louise scoring.30 Participants with a headache score of ≥1 and a Lake Louise Score (LLS) of ≥ 3 were diagnosed with AMS on a daily basis. Participants diagnosed with AMS at least once during the 5 days at high altitude were grouped as having AMS. The LLS is composed of 4 main different subscores: 1) headache, 2) gastrointestinal symptoms, 3) fatigue and/or weakness, and 4) dizziness/light-headedness. The extra subscore, the clinical-functional score, was used to evaluate the overall impact on patients with AMS but is not included in the total score.
Multimodal MR Imaging Acquisitions and Preprocessing
MR imaging was performed using a 3T Discovery MR 750 scanner (GE Healthcare) both at sea level and high altitude to minimize variance caused by scanner differences.
T1-weighted structural MR imaging was acquired using the 3D fast-spoiled gradient recalled protocol with the following parameters: TR = 6.9 ms, TE = 3.0 ms, TI = 450 ms, flip angle = 12°, FOV = 25.6 cm, 188 slices with section thickness = 1 mm, matrix = 256 × 256, voxel resolution = 1 mm, and number of excitations = 1.
rs-fMRI was acquired measuring blood oxygenation level–dependent signals with an EPI sequence: TR = 2000 ms, TE = 30 ms, flip angle = 90°, FOV = 24.0 cm, 41 slices with section thickness = 3 mm, matrix = 64 × 64, voxel resolution = 3.75 mm, and time-series length = 240.
For pseudocontinuous ASL perfusion images, the labeling duration = 1.5 seconds, postlabel delay = 2.0 seconds, TR = 4844 ms, TE = 10.5 ms, TI = 2025 ms, flip angle = 111°, bandwidth = 62.5 kHz, FOV = 24 cm, 36 slices with section thickness = 4 mm, acquisition matrix = 128 × 128, resolution = 1.875 mm, and number of excitations = 3. The proton density–weighted images were obtained with a saturation recovery acquisition with identical parameters.
A summary of the multimodal MR imaging preprocessing pipeline is shown in Fig 1B. We have summarized the preprocessing steps and features extracted for each technique in Table 1. For detailed preprocessing steps, refer to the Online Supplemental Data.
Preprocessing and features of multimodal MR imaging
Prediction Input and Methods
The sea-level MR imaging features were summarized by the mean values in ROIs from the Automated Anatomical Labeling atlas31 (https://www.sciencedirect.com/science/article/pii/S1053811919307803) or the surface atlas32 and were used as prediction input. Sea-level demographic and physiologic features were also entered as potential confounding covariates.
For the prediction methods, we used the Least Absolute Shrinkage and Selection Operator33 logistic regression (LASSO-LR) due to its capability for feature selection (detailed implementation is in the Online Supplemental Data). We applied the leave-one-out framework for cross-validation.
We primarily evaluated the final prediction performance using the area under the curve (AUC) of the receiver operating characteristic (ROC) because of the unbalanced labels (36 AMS of 45 participants). The ROC is suitable for measuring the classification performance on unbalanced labels34⇓-36 because it is generated across a range of decision thresholds and is less sensitive to unbalanced label distribution. Sensitivity and specificity were also calculated at the cut-point of the best Youden index. All prediction methods were implemented using the scikit-learn toolbox, Version 1.0.2 (https://scikit-learn.org/stable/index.html).
Statistical Analyses
For investigating the valid predictors, we performed the feature-selection pipeline shown in Fig 1C. Specifically, we accepted valid predictors that passed both multivariate LASSO-LR and univariate logistic regressions.
T tests were performed to compare image features between groups. Because the predictors are regionally averaged, we further reverified them by detecting group differences between AMS and non-AMS at both voxelwise and network-wise scales, using MR imaging data from both sea level and high altitude.
All features were converted to a standard normal z score before prediction and statistical analyses.
The voxelwise analysis of MR images was performed on the basis of Statistical Parametric Mapping (SPM 12;http://www.fil.ion.ucl.ac.uk/spm/software/spm12). The network-wise t tests between groups used the predefined brain network atlas (https://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation_Yeo2011).37 This atlas was used to re-verify the regional predictors and to detect potential networks involved. A partial Pearson correlation was used to assess the relationship between MR imaging predictors and other brain features they might influence. Before the statistical tests mentioned above, the values were regressed by the covariates of age and sex to exclude their potential effects. The statistical analyses were performed using related in-house scripts in Matlab (MathWorks).
RESULTS
Incidence Rate of AMS
The demographic and physiologic information at sea-level baseline is provided in Table 2, and no significant difference between AMS and non-AMS groups was detected using t tests and χ2 tests.
Demographic and physiologic features for the AMS and non-AMS groups at sea-level baselinea
The overall incidence rate of AMS during 5 days was 80.0% (36 of 45 participants; 95% CI , 66.7%–1.1%). The daily incidence rates of AMS were as follows: 66.7% at 9 hours after exposure (95% CI, 51.1%–80.0%), 48.9% at 22 hours (95% CI, 33.3%–64.4%), 46.7% at 46 hours (95% CI, 31.2%–62.2%), 31.1% at 70 hours (95% CI, 17.8%–46.7%), and 17.8% at 94 hours (95% CI, 6.7%–28.9%). Individual AMS diagnoses are provided in the Online Supplemental Data.
Prediction of AMS Using Multimodal Sea-Level MR Imaging
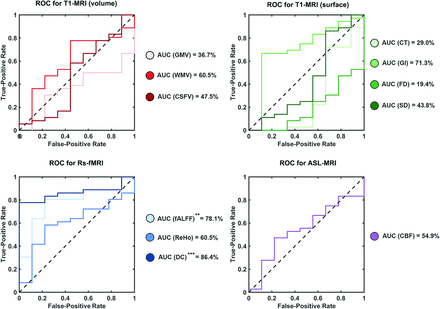
We used each type of feature in ROIs from sea-level multimodal MR imaging to predict AMS at high altitude (Fig 2, and with Youden cutoff in the Online Supplemental Data). Two fMRI metrics showed significant predictive performance: for fALFF as input, AUC = 78.1%, sensitivity = 80.5%, and specificity = 77.8% (P = .0098) and for degree centrality (DC) as input, AUC = 86.4%, sensitivity = 77.8%, and specificity = 100.0% (P = .0008). Other input features had no significant predictive performance, including combined features (such as all fMRI features or all multimodal MR imaging features). The full performance list of all types of input is provided in the Online Supplemental Data.
ROC curve analysis of MR imaging features. The prediction performance using different features of multimodal MR imaging as input, measured by the AUC of the ROC curves. fALFF and DC from fMRI were detected as valid predictors, yielding significant AUC values. CT indicates cortical thickness; FD, fractal dimension; GI, gyrification index; GMV, gray matter volume; ReHo, regional homogeneity; SD, sulcus depth; WMV, white matter volume, CSFV, CSF volume. ** P < .01, *** P < .001.
Identification and Verification of Sea-Level MR Imaging Predictors
Following the predictions, sea-level predictors were identified from the multivariate LASSO-LR. Then, these potential predictors were re-verified by univariate logistic regression, and only those that passed both regressions were regarded as valid predictors. Valid predictors were fALFF and DC values in the regions listed in Table 3.
ORs of significant predictors for AMS, selected by both the LASSO-LR and univariate logistic regression
Among the valid predictors, risk factors with ORs > 1 were mainly located in the orbitofrontal cortex and the right supramarginal gyrus. Protective factors with odds ratios < 1 were mainly located in the paracentral lobule, supplementary motor area, and Rolandic operculum, all of which belonged to the somatomotor network (SMN). The left superior and middle temporal gyri were also included as protective factors, which were near the Rolandic operculum. These predictors selected by the LASSO-LR models remained robust during the leave-one-out cross-validation (Online Supplemental Data).
Predictors of AMS Present in the SMN Both at Sea Level and High Altitude
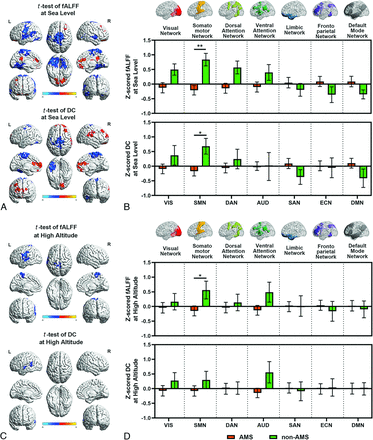
To further verify the region-wise predictors, we performed voxelwise t tests between groups on the fMRI metrics (fALFF and DC) maps. Both at sea level and at high altitude, the AMS group showed weaker functional metrics mainly in the motor and sensorimotor areas (Fig 3A, -C) than the non-AMS group (under P < .05, corrected by Gaussian random field).
Comparison of functional metrics between AMS and non-AMS groups. A, Sea-level voxelwise differences. Differences between AMS and non-AMS groups are identified using t tests, corrected with the Gaussian random field at P < .05. For both fALFF and DC, AMS showed increased functional metrics in the medial prefrontal cortex, supramarginal gyrus, posterior cingulate cortex, and superior frontal gyrus and decreased functional metrics in the supplementary motor area, postcentral gyrus, paracentral lobule, Rolandic operculum, middle cingulate cortex, and superior temporal gyrus. B, Sea-level network-wise differences. Among 7 typical functional networks at sea level, we detected significant lower fALFF and DC in the SMN in the AMS group (*: P < .05; **: P < .01). The error bars present the standard error of the mean (SEM). C, High-altitude voxelwise differences. At high altitudes, for both fALFF and DC, AMS showed decreased functional metrics in the postcentral gyrus, Rolandic operculum, and superior temporal gyrus. D, High-altitude network-wise differences. Among 7 typical functional networks at high altitude, we detected significant lower fALFF in SMN in the AMS group (*: P < .05). The error bars present SEM. L indicates left; R, right.
We further verified the region-wise predictors by detecting network-wise group difference on the z-scored fMRI metric (fALFF and DC) maps using a predefined atlas.37 At sea level (Fig 3B), the significant functional group difference in the predefined SMN between the AMS and non-AMS groups was detected by t tests: For fALFF, the AMS group showed significantly (P = .0082) lower values than the non-AMS group in the SMN (mean AMS) = −0.2095 (SD, 0.1613), mean (non-AMS) = 0.8381 (SD, 0.2140), group difference = 1.0476 (95% CI , 0.3590–1.7362). Similarly, for DC, the AMS group had significantly (P = .0424) lower values than the non-AMS group in the SMN (mean AMS) = −0.1706 (SD, 0.1632), mean (non-AMS) = 0.6824 (SD, 0.2693), group difference = 0.8529 (95% CI, 0.1394−1.5665). At high altitude (Fig 3D), for fALFF, the AMS group showed significantly (P = .0320) lower values than the non-AMS group in the SMN (mean AMS) = −0.1486 (SD, 0.1685), mean (non-AMS) = 0.5615 (SD, 0.3019), group difference = 0.7101 (95% CI, −0.0227−1.4429). No significant group differences were observed in networks other than the SMN under the threshold of P = .05.
AMS Features Associated with the SMN at High Altitude
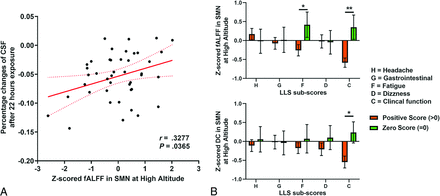
Using the multimodal MR imaging scanned at high altitude, we further identified the influence of fMRI predictors (fALFF and DC in SMN) by performing the partial Pearson correlation between fMRI predictors and other modalities of brain MR imaging at high altitude (including the whole-brain GM, WM, CSF, and CBF). At 22 hours after exposure, the fALFF in the SMN was significantly correlated with the percentage changes of CSF volume (r = 0.3277 and P = .0365, shown in Fig 4A). Other brain components except CSF were also examined but showed no significance; these are listed in the Online Supplemental Data.
The pathophysiologic impact of sea-level functional predictors on AMS, measured at 22 hours after high-altitude exposure. A, Correlation between the fALFF in the SMN and the percentage change in CSF volume. The partial Pearson correlation coefficient is r = 0.3277 and P = .0365, considering the effects age and sex. B, T test comparisons show significant differences in fALFF and DC in the SMN between participants having positive LLS subscores and those with zero subscores. The error bars present standard error of the mean. The LLS subscores associated with SMN function were fatigue and clinical function scores. * P < .05; **P < .01.
Moreover, we examined the relationship between fMRI predictors in the SMN and 5 different LLS subscores, which were all measured immediately before MR imaging and at 22 hours at high altitude. In Fig 4B, by performing the t tests, we found that the group that showed significantly (P = .0460) positive fatigue symptoms had lower fALFF in the SMN (mean positive LLS) = −0.2578 (SD, 0.1523), (mean negative LLS) = 0.4186 (SD, 0.3341), group difference = 0.6764 (95% CI,−0.0136−1.3664), and the group whose clinical function was affected had significantly (P = .0024) lower fALFF (mean positive LLS) = −0.5836 (SD, 0.1265), (mean negative LLS) = 0.3525 (SD, 0.3214), group difference = 0.9361 (95% CI, 0.3403−1.5320), and significantly (P = .0112) lower DC, (mean positive LLS) = −0.5452 (SD, 0.1612), (mean negative LLS) = 0.2380 (SD, 0.2799), group difference = 0.7832 (95% CI, 0.0856−1.4808) in the SMN.
DISCUSSION
Although a previous MR imaging study for AMS28,29 had detected several brain abnormalities in the diffusion MR imaging after exposure, to the best of our knowledge, our study was the first to perform multimodal MR imaging at sea level and at high altitude for both integrated predicting and mechanistic investigating of AMS. The major findings lie in 2 parts: First, we discovered that sea-level SMN function can predict AMS with a high performance of AUC = 86.4%, which offered a method to predict the AMS risk for individuals requiring rapid ascent to high-altitude areas, such as those undertaking urgent high-altitude missions. Second, we further verified that the predictors also showed significant group difference between AMS and non-AMS at high altitude. The predictors were also associated with the changes in CSF volume and the extent of fatigue and clinical function.
These findings suggest that a cerebral functional basis for AMS can be detected early at sea level and shows promise as a noninvasive and accurate prediction tool and can offer insight into the neural basis of the susceptibility and development of AMS.
Methodologic Considerations for the Reliability of Prediction
We used LASSO-LR for its capability for feature selection (Online Supplemental Data). The selected features remained robust in cross-validation and different coefficient thresholds (Online Supplemental Data). We did not correct multiple comparisons among predictors because we used a data-driven LASSO-LR but did not screen all features. The multiple-comparisons correction might unnecessarily increase the risk of type II errors (false-negatives), thereby hindering the exploring of meaningful predictors. Despite including ASL MR imaging and structural MR imaging at both sites, our exploratory analyses rarely identified significant predictors from these modalities. We observed several ROC curves significantly below the diagonal line. This finding suggests that while these modalities may hold some useful information, they fail to provide robust and reliable predictions, indicating their limited applicability in this context.34,38 The combined features like all fMRI or all MR imaging features showed insignificant performance (Online Supplemental Data), which indicates that simply combining all features into the prediction model will lead to overfitting and that each type of feature should be tested independently.
AMS Predictors as the Functional Features in the SMN
We found that functional features of fALFF and DC in the SMN were significant predictors for AMS (Table 3). We used both voxelwise and network-wise t tests to re-verify these predictors, as shown in Fig 3. The SMN in the predefined brain network atlas37 broadly includes the primary motor and somatosensory cortices as well as part of the superior temporal gyrus, and these regions match the protective predictors well (Table 3).
For the fALFF and DC as predictors, higher DC in the SMN indicates that the SMN plays a more critical role in facilitating communication and information flow among other brain regions, while higher fALFF in the SMN means stronger spontaneous neural activity.39,40 Insignificant regional homogeneity results suggested that AMS might be less related to local synchrony within brain regions. Because weaker SMN function leads to higher AMS probability in this study, resting-state sea-level SMN could potentially indicate the capability for subsequent high-altitude adaptation, showing promise for clinical use. The stronger SMN activity for better adaptation aligns with previous studies, which regard the SMN as regions responsible for integrating sensory signals from the environment, especially for the sensation of hypoxia,41⇓-43 while hypoxia is a commonly-recognized trigger of AMS.1 Moreover, the SMN is found to be specifically affected by the brain hypoxia–ischemia injury in the former reports on neonatal rats and humans;44⇓-46 this finding can also be related to the important role of SMN during the high-altitude hypoxia and the onset of AMS. In summary, we speculated that a functionally more active SMN could imply a more efficient processing and integration of sensory input, indicating protective and adaptive compensatory mechanisms in response to exposure.
The risk factors of AMS in Table 3 and Fig 3A were mainly distributed in the orbitofrontal cortex and right supramarginal gyrus, aligning with the sensory processing function that was previously proposed.47,48 However, these group differences did not remain significant at high altitude (Fig 3C). Therefore, this study focused more on the SMN as the most promising protective predictor of AMS.
Identifying the Pathophysiologic Impact of AMS Predictors at High Altitude
At high altitude, a weaker SMN function showed a significant association with less change of CSF volume after exposure (Fig 4A). Other modalities of GM, WM, and CBF showed no correlation with SMN function (Online Supplemental Data). Changes in CSF and the saturation of pulse oxygen (SpO2) are shown in the Online Supplemental Data. This result provides evidence for the differential effects of various brain components at the onset of high-altitude symptoms.25,27 On the basis of the high-altitude MR imaging, our results propose that among the volume effect of different brain components,19 CSF is directly related to brain functional activity during AMS, a relationship also observed in a previous study,49 and we propose a potential mechanism of AMS susceptibility: SMN-based functional predictors might be dynamically associated with AMS by reflecting changes in CSF flow after exposure.
Then, we detected the differences of SMN function between LLS-positive and -negative groups (Fig 4B). Although headache is widely believed to be the key factor of AMS,2 our t test analyses suggest that the SMN function might lead to the development of AMS by influencing the levels of fatigue and the clinical function. In detail, the clinical function measures the overall influence extent of the symptoms on the individual’s activity. The relationships between weaker SMN function and fatigue or clinical function was formerly confirmed in studies without high-altitude exposure50,51 and was in line with our results. However, our results further suggest that this mechanism can underlie the onset of AMS.
Overall, we suggest that the functional features of the SMN within the brain serve as early indicators and pathologic features of AMS, which suggests focusing on the functional aspects of the brain in future AMS research.
Potential Clues for Future Treatment and Prevention of AMS
Our SMN-based hypothesis on AMS susceptibility could potentially explain why lower-altitude or hypoxic training before exposure is effective in preventing AMS,52 as interpreted by the augmentation of SMN functionality during training, because this augmentation was also observed in training patients with incomplete spinal cord injury.53 Additionally, the relationship between SMN activity and CSF dynamics or LLS subscores might suggest a potential treatment target. Treatments that modulate CSF volume and flow in response to changes in clinical function or fatigue state could be explored during the development of AMS.
Limitations
Our study had limitations. First, we defined AMS as any diagnosis during the 5 time points at the plateau to achieve a generic prediction for wider populations, but future studies can consider the different onset, duration, and extent of AMS as separate subgroups. Second, all participants are sea-level residents with ages relatively limited to 20–40 years (mean, 28.7 [SD, 4.4] years). Therefore, the generalizability to other age ranges and to residents at high altitude is relatively weaker. However, we selected this age range to minimize the potential confounding effects of age-related brain changes.
CONCLUSIONS
Our multimodal MR imaging study revealed that the SMN function, as detected by sea-level rs-fMRI, emerged as a crucial predictor for AMS among multimodal MR imaging features. Furthermore, we validated its pathophysiologic impact at high altitudes. Although this study is derived from a relatively small sample size of 45 and should be considered as a preliminary feasibility study that requires validation in larger and more diverse populations, these significant findings offer a potential direction for developing a screening tool to predict AMS, particularly for individuals requiring rapid ascent to high-altitude areas, such as those undertaking urgent high-altitude missions, and also offer insight into the underlying mechanisms of AMS at high altitudes.
Footnotes
W. Zhang and J. Feng contributed equally to this work.
This work was supported by National Natural Science Foundation of China, grant No. 81741115, 81771923, 11975249, and 12175268, Chinese Military Creative Project, grant No. 16CXZ014, Chinese Military Healthcare, grant No. 16BJZ11.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 20, 2023.
- Accepted after revision January 23, 2024.
- © 2024 by American Journal of Neuroradiology