Abstract
BACKGROUND AND PURPOSE: Tumor embolization through the meningohypophyseal trunk and inferolateral trunk is known to be effective in skull base tumors; however, microcatheter cannulation into these arteries is difficult, and the number of cases that can be safely embolized is limited. In this study, we present a novel embolization procedure for the meningohypophyseal trunk and inferolateral trunk using the distal balloon protection technique and detail its clinical efficacy and complication risks. We developed this procedure to allow safe embolization in patients who cannot be adequately cannulated with microcatheters into these arteries.
MATERIALS AND METHODS: Patients who underwent meningohypophyseal trunk or inferolateral trunk embolization using the distal balloon protection technique for skull base tumors at our institution between 2010 and 2023 were included. In this procedure, the ICA was temporarily occluded with a balloon at the ophthalmic artery bifurcation, the microcatheter was guided to the meningohypophyseal trunk or inferolateral trunk vicinity, and embolic particles were injected into the arteries. The balloon was deflated after the embolic particles that had refluxed into the ICA were aspirated.
RESULTS: A total of 25 meningohypophyseal trunks and inferolateral trunks were embolized during 21 operations. Of these 25 arteries, only 9 (36.0%) were successfully cannulated with microcatheters. Nevertheless, effective embolization was achieved in all cases. Permanent complications occurred in only 1 case (4.8%) in which the central retinal artery was occluded during inferolateral trunk embolization, resulting in a visual field defect. No permanent complications resulting from the embolic cerebral infarction were observed. Of 16 cases that underwent MR imaging within a week after embolization, however, 11 (68.8%) demonstrated embolic cerebral infarctions.
CONCLUSIONS: In patients with skull base tumors with meningohypophyseal trunk or inferolateral trunk feeders that cannot be catheterized directly, embolization using the distal balloon protection technique for tumor supply can be considered as a salvage technique.
ABBREVIATIONS:
- AC
- aspiration catheter
- FR
- flow reverse
- GC
- guide catheter
- ILT
- inferolateral trunk
- MHT
- meningohypophyseal trunk
Preoperative embolization is known to be effective against extra-axial tumors and is a widely accepted procedure.1⇓⇓⇓⇓⇓⇓⇓⇓⇓-11 It is known that preoperative tumor embolization is particularly effective for skull base tumors because tumor-feeding arteries are located at the deepest part of the surgical field, making devascularization of feeding arteries difficult until the tumor has been removed.2,7,8,12
However, preoperative embolization of skull base tumors presents several challenges.13 In particular, when embolizing the meningohypophyseal trunk (MHT) and inferolateral trunk (ILT), reflux of embolic particles into the ICA has been reported.2,4,7,10 This is because the MHT and ILT are usually very tortuous, making microcatheter cannulation into these arteries difficult.2,8 When the MHT and ILT are aggressively embolized, neurologic complications are reported to occur in 22.1% and 13.3% of cases, respectively.9 On the other hand, MHT and ILT embolization is safe provided that cannulation is performed reliably,4,7,8,10 though the number of patients who can be adequately catheterized into the MHT and ILT is limited. In a report of skull base meningioma embolization with safety being the highest priority, only 9 of 28 MHTs (32.1%) and 1 of 5 ILTs (20.0%) were embolized,10 indicating that safe tumor embolization via these arteries can be performed in only a limited number of cases.
To solve this problem, we developed a novel embolization procedure that combines a distal balloon protection technique.2 In this procedure, the MHT and ILT are embolized while occluding the ICA with a balloon, and embolic particles that have refluxed into the ICA are aspirated and removed. This technique allows embolization of the MHT and ILT, even if they are difficult to cannulate using microcatheters. Our current report explores the efficacy and safety of MHT and ILT embolization using the distal balloon protection technique.
MATERIALS AND METHODS
Study Design and Population
We retrospectively analyzed all patients who underwent tumor embolization via the MHT and/or ILT for extra-axial skull base tumors using the distal balloon protection technique at our institution between February 2010 and March 2023. Extra-axial skull base tumors were defined as those attached to the dura mater located in the anterior fossa, cavernous sinus, sphenoid ridge, middle fossa, petroclival region, cerebellopontine angle, foramen magnum, or tentorium.
Data Collection.
Patient information such as age, sex, tumor location, and pathology, as well as postembolization complications, was collected using medical records and pre- and postoperative CT and/or MR imaging. Neurologic findings immediately after tumor embolization, head CT performed immediately after tumor embolization, and MR imaging performed within 1 week of embolization were used to ascertain the occurrence of complications. The extent of tumor resection was determined using MR images obtained within 3 months after surgery and was divided into 3 grades: gross total resection, complete resection of the tumor mass; subtotal resection, complete resection of the tumor mass except for a part of the tumor adjacent to critical structures and a tumor invasion part into the dural sinuses; and partial resection, other than gross total resection and subtotal resection. In addition, angiograms and embolization operative records were reviewed to determine the surgical instrument used, artery embolized, and embolization efficacy. The latter was determined by 2 endovascular surgeons on the basis of postoperative ICA angiography findings of complete, partial, or no disappearance of tumor staining from the embolized artery. Finally, the operative records of tumor resection were reviewed to determine blood loss.
Evaluation of Postoperative Cerebral Infarction.
The presence of postoperative cerebral infarction was evaluated through an MR imaging performed within 1 week following embolization. Cerebral infarction was diagnosed on the basis of the presence of high-intensity areas observed on DWI. The number and size of the cerebral infarction were evaluated using the DWI grading scale, as outlined in previous reports: grade A, no high-intensity areas; grade B, minor high-intensity areas (≤5 spots, with each spot ≤10 mm); grade C, some small high-intensity areas (>6 spots, with each spot ≤10 mm); grade D, large high-intensity areas (at least 1 spot of >10 mm).14
Surgical Procedure of Embolization Using Distal Balloon Protection.
The indication for tumor embolization was determined by a discussion between the neurosurgeon performing the tumor resection and the endovascular surgeon on the basis of tumor angiographic findings. Tumor embolization was performed primarily for tumors that were large and rich in blood flow; it was expected that it would be difficult to devascularize the feeding artery during tumor resection.
Tumor embolization was performed 1 day before tumor resection. All embolization procedures were performed with 7F- or 8F-equivalent guide catheters (GCs). Initially, the GC was guided via the right femoral artery into the ICA, and a balloon was guided near the ophthalmic artery bifurcation. Next, microcatheter cannulation was attempted into the MHT or ILT. If cannulation was difficult, the microcatheter was guided as closely as possible to the orifice of the MHT or ILT. After the ICA was occluded with a balloon at the bifurcation of the ophthalmic artery, the embolic particles were injected through a microcatheter into the MHT or ILT (Fig 1). During ICA occlusion, care was taken to ensure that the orifice of the ophthalmic artery was occluded to prevent the migration of embolic particles into the ophthalmic artery. The temporary occlusion of the ICA during embolization was limited to 10 minutes. After we confirmed that a sufficient embolic effect had been achieved, the embolic particles that had refluxed into the ICA were aspirated and removed using 3 methods.
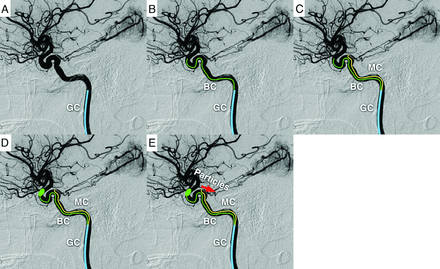
Schematic representation of tumor embolization using the distal balloon protection technique. First, a GC is placed in the ICA (A). Then, the balloon catheter is guided to the proximity of the bifurcation of the ophthalmic artery (B). Next, a microcatheter is inserted into the MHT or ILT (C). If cannulation is difficult, the microcatheter is guided near the orifice of the MHT or ILT. The balloon is then inflated to occlude the internal carotid and ophthalmic arteries (D). The embolic particles are injected from the microcatheter into the MHT or ILT (E). BC indicates balloon catheter; MC, microcatheter.
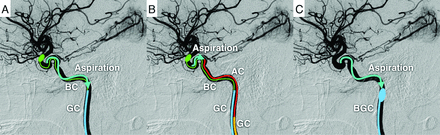
The first method involved aspirating and removing the embolic particles from only the GC (GC method) (Fig 2A). In this method, the embolic particles were aspirated and removed using the GC after embolization. In the GC method, aspiration from the GC was performed at least 3 times, even if no embolic particles were identified; if embolic particles were identified, aspiration was repeated until no embolic particles were present.
Schematic representation of aspiration and removal of embolic particle reflux into the internal carotid artery. In the GC method, embolic particles are aspirated and removed using the CG, while the ICA is occluded with a balloon (A). In the AC method, a GC different from the one used for embolization is placed in the common carotid artery (for convenience, the GC is depicted in the ICA), and the AC is guided to the vicinity of the meningohypophyseal or inferolateral trunk to aspirate and remove embolic particles (B). In the AC and FR method, after aspiration and removal of embolic particles by the aspiration method, the balloon GC is inflated to reverse blood flow in the ICA to remove embolic particles more reliably (C). BC indicates balloon catheter; BGC, balloon guide catheter.
The second method involved aspiration and removal of embolic particles using an aspiration catheter (AC) (AC method) (Fig 2B). In this method, apart from the GC used for embolization, a 6F- or 7F-equivalent GC was guided via the left femoral artery into the common carotid artery, and an AC was guided to the vicinity of the balloon to aspirate and remove the embolic particles that had refluxed into the ICA. Notably, when the ICA was occluded by using a PercuSurge Guidewire (Medtronic), no additional GC was inserted. In the AC method, similar to the GC method, aspiration from the AC was performed at least 3 times even if no embolic particles were identified; if embolic particles were identified, aspiration was repeated until no embolic particles were present.
In the third method, after the embolic particles were aspirated and removed by the AC method, the cervical ICA was further occluded with a balloon GC, the balloon on the distal side was deflated to allow the ICA to flow back, and the embolic particles were drained from the ICA via the balloon GC (AC and flow reverse [FR] method) (Figs 2C and 3). In the AC and FR method, aspiration from the balloon GC was completed once if no embolic particles were identified; however, if embolic particles were identified, aspiration was repeated until no embolic particles were present.
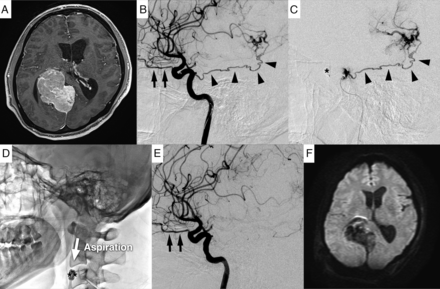
A case of tumor embolization via the MHT using the distal balloon protection technique. Preoperative tumor embolization was performed for a right falcotentorial meningioma through the right MHT (A and B). In this case, the microcatheter was guided to the vicinity of the orifice of the MHT because cannulation of the microcatheter into the MHT was not possible (C). Next, the ICA and ophthalmic artery were occluded with a balloon (C), and the MHT was embolized with embolic particles. After embolization, the AC was guided to the vicinity of the MHT to aspirate and remove the embolic particles. This step was followed by inflating the balloon GC and deflating the balloon catheter to reverse blood flow in the ICA to remove the embolic particles (D). Finally, complete embolization of the MHT was confirmed (E). Postoperative MR imaging showed no embolic cerebral infarction (F). The black arrow indicates the ophthalmic artery; black arrowhead, MHT; single asterisk, balloon catheter; double asterisk, balloon GC.
In all 3 methods, aspiration was performed after embolization using a 20-mL syringe, and continuous aspiration during embolization was not performed.
Ethical Consideration.
This retrospective study was approved by our institutional review board (Fujita Health University, protocol No.: HM23-123). All patients provided written informed consent for treatment, and we offered an opt-out approach for participation.
In this study, 3 different embolic particles were used (Embosphere [trisacryl gelatin microspheres], Merit Medical Systems); Ivalon [polyvinyl alcohol], Ivalon; and Avitene [microfibrillar collagen], Zeria Pharmaceutical). Before the introduction of Embosphere, there were no approved embolic particles for tumor embolization in our country. Consequently, before the availability of Embosphere, Ivalon or Avitene were used as off-label embolic particles following the acquisition of written informed consent from the patient.
RESULTS
Patient Characteristics and Surgical Procedures
A total of 21 tumor embolization procedures via the MHT and/or ILT with the distal balloon protection technique were performed in 19 patients with extra-axial skull base tumors during this study. Two of the 19 patients underwent tumor embolization twice. The median age at surgery was 47 years (range, 21–68 years), with 13 women and 6 men. Of the 19 patients, 17 had meningiomas and 2 had solitary fibrous tumors. The meningioma subtypes were meningothelial in 8, angiomatous in 5, secretory in 2, chordoid in 1, and difficult to classify (diagnosed as World Health Organization grade 1 equivalent) in 1 patient. The tumor attachment sites were the petroclival in 13, falcotentorial in 3, sphenoid ridge in 2, and cerebellopontine angle in 1 patient.
A total of 19 MHTs and 6 ILTs were embolized using 21 embolization procedures. Of these embolized arteries, 8 MHTs (42.1%) and 1 ILT (16.7%) were successfully cannulated using microcatheters. Particles were used as the embolic material in all surgeries; Embosphere (100–300 μm or 300–500 μm) was used in 12 embolization procedures, Ivalon was used in 5, and Avitene was used in 4. Avitene used in this study was supplied in powder form, and a pinch of Avitene was dissolved in 20 mL of contrast medium for the embolization procedure. As for the method of aspiration and removal of embolic particles, the GC method was used in 3 embolization procedures; the AC method, in 6; and the AC and FR method, in 12 (Online Supplemental Data).
Efficacy and Complications of Embolization
Regarding the efficacy of tumor embolization, complete disappearance of tumor stain was achieved in 14 of 19 MHTs (73.7%); and partial disappearance, in the remaining 5 (26.3%). Complete disappearance of the tumor stain was observed in 4 of 6 ILTs (66.7%); and partial disappearance, in the remaining 2 (33.3%). An embolic effect was observed in all cases. When the embolization effect was examined on the basis of whether the microcatheter could be cannulated into the ILT or MHT, the complete disappearance of the tumor stain was confirmed in 6 of the 9 arteries (66.7%) that could be cannulated and in 12 of the 16 arteries (75.0%) that could not (Online Supplemental Data).
Two of the 21 embolization procedures (9.5%) exhibited symptomatic complications associated with embolization of the ILT: transient hemiparalysis associated with embolic cerebral infarction in one case and visual field disturbance due to central retinal artery occlusion in the other. Permanent sequelae were observed in only 1 case (4.8%) of the above-mentioned visual field disturbance (Online Supplemental Data).
Regarding the postoperative imaging evaluation, all patients underwent CT immediately after embolization, and 16 of the 21 patients underwent MR imaging within 1 week after embolization. Postoperative MR imaging was performed in 1 of 3 cases using the GC method, in 4 of 6 cases using the AC method, and in 11 of 12 cases using the AC and FR method. Postoperative CT revealed no complications in any of the patients. However, MR imaging confirmed embolic cerebral infarction in 11 of 16 patients (68.8%): in 1 patient (100%) using the GC method, in 2 of 3 (50.0%) using the AC method, and in 8 of 11 (72.7%) using the AC and FR method. The details of the DWI grading scale were the following: grade A in 5 patients (31.3%), grade B in 2 (12.5%), grade C in 8 (50.0%), and grade D in 1 (6.3%). Of these 11 cerebral infarctions, 1 patient with the GC method had transient hemiparalysis as described above, whereas 10 patients with the AC and AC and FR method were asymptomatic. According to the DWI grading scale, the patient with a grade D experienced transient paralysis, whereas 10 patients with grades B and C were asymptomatic. No cranial nerve ischemia occurred in any patient.
In 1 of the 12 patients with the AC and FR method in which the ILT was embolized, occlusion of the central retinal artery occurred, and the embolic particles were thought to have migrated into the central retinal artery through the anastomosis between the ILT and ophthalmic artery or the remaining embolic particles in the ICA migrated directly into the central retinal artery.
Tumor Resection
Tumor resection was successfully performed in all cases. Gross total resection was achieved in 2 cases; subtotal resection, in 9 cases; and partial resection, in 10 cases. The median blood loss during surgical resection was 212 mL (range, 30–848 mL). There were no cases in which tumor resection was interrupted due to difficulty in hemostasis. No major surgery-related complications or deaths occurred.
Representative Case (Surgical No. 10)
A man in his 30s presented with symptoms including headache, visual field disturbance, and cognitive dysfunction. MR imaging revealed an extra-axial skull base tumor with an attachment from the falx to the right cerebellar tent (Fig 3A). Preoperative ICA angiography showed a prominent tumor stain from the right MHT (Fig 3B), and tumor embolization from the MHT with the AC and FR method was performed 1 day before tumor resection (Online Supplemental Data).
To begin the embolization procedure, we initially guided a 7F balloon GC (OPTIMO EPD; Tokai Medical Products) into the right ICA via the right femoral artery. Next, a sheathless 5F GC (ASAHI FUBUKI Dilator kit; Asahi Intecc) was guided via the left femoral artery to the right common carotid artery for particle aspiration.
Efforts were made to cannulate the microcatheter (Excelsior 1018; Stryker) and microguidewire (Glidewire GT, 0.016-inch double angle; Terumo Interventional Systems) into the MHT via a 7F balloon GC, but these attempts were unsuccessful. Therefore, the apex of the microcatheter was redirected to the orifice of the MHT, and the embolic particles (Embosphere 300–500 μm) were injected toward the orifice after the temporal occlusion of the ICA at the origin of the ophthalmic artery was achieved using a microballoon catheter (Magic B1; Balt) through the 7F balloon GC (Fig 3C).
Immediately following embolization, the AC (Thrombuster; Kaneka Medix) was guided through a sheathless 5F GC to the vicinity of the balloon to aspirate and remove the remaining embolic particles in the ICA. In addition, the balloon GC was inflated to obstruct blood flow at the cervical ICA, and the distal microballoon catheter was deflated to reverse the blood flow in the ICA and drain the remaining embolic particles out of the body through the balloon GC (Fig 3D). Finally, the complete disappearance of tumor stain from the MHT was confirmed (Fig 3E). There were no new neurologic symptoms following embolization, and postoperative MR imaging revealed no evidence of embolic infarction (Fig 3F).
The tumor was resected using a posterior interhemispheric approach (operation time: 949 minutes, blood loss: 498 mL), and the patient was discharged without any postoperative complications. The pathologic diagnosis confirmed the presence of a solitary fibrous tumor.
DISCUSSION
To our knowledge, this is the first study to evaluate the efficacy and safety of tumor embolization via the MHT and ILT using the distal balloon protection technique. The distal balloon protection technique presented in this study can embolize essentially all MHTs and ILTs because cannulation into these arteries is not required and the incidence of permanent complications was 4.8%. Only 32.1% of MHTs and 20% of ILTs can be embolized when catheter cannulation is prioritized in conventional surgery, whereas complications occur in 22.1% of MHT embolizations and 13.3% of ILT embolizations when aggressive embolization is performed.9,10 The embolization of the MHT and ILT using the distal balloon protection technique greatly expanded the surgical target compared with conventional methods.
Embolization Efficacy
The results of this study showed that tumor embolization effects were observed after all operations. Even when microcatheter cannulation into the MHT or ILT was difficult, tumor stains completely disappeared in 75% of the cases, suggesting that even without cannulation, injecting embolic particles toward the target artery provides a sufficient tumor embolization effect. We have named the tumor embolization technique with distal balloon protection the “para-para method” (from the Japanese word “para-para,” which means sprinkle) because of sprinkling the embolic particles into the tumor. Although safety issues remain, the para-para method presented here allows tumor embolization via all MHTs and ILTs; this result is expected to significantly expand the indications for tumor embolization via MHT and ILT. Surgical instruments developed in recent years may facilitate the selection of these arteries. Arterial embolization after selecting these arteries is the first choice; however, tumor embolization using the para-para method is useful as a second option when these arteries cannot be selected.
In the present study, gross total resection was achieved in only 2 cases. Because tumor embolization in this study was performed on tumors with large and abundant blood flow, the tumor part adjacent to the brainstem, eloquent cerebral cortex, cranial nerves, and critical arteries and veins, and invading the dural sinuses tended to be unresectable. Conversely, these results indicate that tumors with a high risk of complications were primarily included in the present study. Because tumor resection rates are determined by various factors, it is difficult to evaluate the efficacy of tumor embolization solely on the basis of tumor resection rates. On the other hand, the fact that there were no cases in which hemostasis was difficult to achieve during resection may reflect the effectiveness of tumor embolization.
Methods for Aspiration and Removal of Embolic Particles and Risk of Embolic Cerebral Infarction
Three methods were used to remove the embolic particles in this study; however, only the GC method caused symptomatic embolic cerebral infarction (grade D on the DWI grading scale). This result is presumably because the GC method can only aspirate blood around the GC and thus cannot sufficiently aspirate embolic particles. In contrast, the AC and AC and FR methods did not cause symptomatic cerebral infarction. While we used these methods, only minor cerebral infarctions of grade B or C on the DWI grading scale were observed. This issue may be because the aspiration of blood in the vicinity of the orifice of the MHT and ILT allowed sufficient aspiration and removal of embolic particles that refluxed into the ICA. However, a high frequency of postoperative embolic cerebral infarction has been observed even when the AC or AC and FR method was used. Embolization using the para-para method should be considered as an alternative when direct catheterization into the MHT and ILT is difficult. Compared with the AC method, the AC and FR method is presumed to be more reliable for aspirating and removing embolic particles, given the nature of the procedure. Because embolic infarction was confirmed in more than one-half of the cases in the present study, it would be preferable to use the AC and FR method, which more reliably removes embolic particles. In addition, distal access catheters having the same capability as aspiration catheters have been developed recently. Although the present study did not include a case with a distal access catheter, it is likely that a coaxial system with a distal access catheter could be used to aspirate embolic particles in a similar manner through a single access route.
Risk of Complications Other Than Cerebral Infarction
In addition to the aforementioned embolic cerebral infarction, other complications of the MHT and ILT have been reported, including occlusion of the vasa nervorum of the cranial nerves and the risk of migration of embolic particles into normal arteries via dangerous anastomoses.4,7,8,10
Although no occlusion of the vasa nervorum occurred in this study, the choice of embolic material is known to be important for avoiding occlusion.4,5,7,8,10 The vasa nervorum diameter is usually less than 100–150 μm,5,15 and avoiding the use of liquid embolic material while using embolic particles of >100 μm is important to avoid its occlusion.5,8 Conversely, tumor embolization is more effective when smaller-diameter embolic particles are used, which can reach the tumor more easily.7 Considering that tumor embolization is only an adjunctive therapy for tumor removal, it is necessary to avoid complications as much as possible. Although the extent of tumor stain and the location of tumor-feeding arteries can vary, it seemed reasonable to select embolic particles at least larger than the Embosphere 100–300 μm to balance the benefits obtained and the risk of complications, given that no cranial nerve disturbance occurred in this study.
In addition to the vasa nervorum, dangerous anastomosis should also be noted during embolization of the MHT and ILT.8 Particular attention should be paid to the anastomosis with the ophthalmic artery via the deep recurrent ophthalmic artery during embolization of the ILT.8 In the present study, occlusion of the central retinal artery occurred in 1 patient. Although it is conceivable that the remaining embolic particles in the ICA migrated directly to the central retinal artery, this dangerous anastomosis could have been the cause. In the aforementioned case, the microcatheter could not be cannulated into the ILT; therefore, the ILT could not be fully evaluated. Given the magnitude of the impact of visual field defects, it may be appropriate to avoid embolization from the ILT if the dangerous anastomosis cannot be adequately evaluated. Furthermore, because dangerous anastomoses that were not initially identified may be found after embolization,7 it is necessary to carefully consider the surgical indications for ILT embolization, especially considering the risk of blindness and the benefits of embolization. In the present study, the balloon was inflated at the ophthalmic artery bifurcation to prevent the embolic particles from migrating into the ICA via the ophthalmic artery if they flowed back into the external carotid artery; however, because the ICA cavernous portion has various dangerous anastomosis, inflating the balloon immediately distal to the artery to be embolized may further reduce the risk of the embolic particles migrating into the dangerous anastomosis.
Appropriateness of MHT and ILT Embolization
It is difficult to rigorously evaluate the clinical efficacy of tumor embolization because evaluating individual tumors identically is challenging and studies on tumor embolization are usually strongly influenced by neurosurgeon and endovascular surgeon biases.10 Therefore, tumor embolization, especially from arteries posing a risk for complications, should be decided carefully. The decision to perform tumor embolization in this study was ultimately made through discussion between the neurosurgeon and the endovascular surgeon; however, the basic selection was focused on tumors with large, abundant blood flow and difficulty in feeding artery devascularization. Consequently, tumors with rich blood flow, such as angiomatous meningiomas, chordoid meningiomas, and solitary fibrous tumors, were frequently embolized in this study. Although these hemorrhagic tumors accounted for approximately one-half of the cases in the present study, the median amount of bleeding was only 212 mL, which is less than that in previous reports,7 suggesting that tumor embolization was sufficiently effective.
There may be some controversy regarding the 4.8% permanent complication rate and the high frequency of postoperative embolic infarction shown in this study. Preoperative embolization is only an adjunctive procedure to tumor resection, and a lower complication rate will be required. On the other hand, given the difficulty of resecting skull base tumors, the para-para method may be viewed favorably by neurosurgeons performing tumor resections because it may reduce the risk of surgical complications,13 increase the resection rate,16 and improve the progression-free survival.17 Even today, surgical complications and postoperative neurologic deficits related to tumor resection are common, particularly in petroclival meningiomas, which often have feeders branching from the MHT and ILT.18 The worsening of cranial nerve deficits after tumor resection has been reported to occur in 23% to 76% of patients,18⇓⇓⇓⇓⇓⇓-25 and the worsening of extremity weakness occurs in approximately 10%.18,26 Furthermore, even in a series in which the gross total resection rate was kept as low as 15.4% via combination with postoperative radiation therapy, as many as 16.9% of patients had severe complications due to stroke or intracranial hematoma after tumor resection.18 These reports indicate that the removal of a petroclival meningioma, which is often the subject of the para-para method, is still challenging. Tumor embolization using the para-para method should be considered as a salvage technique when direct catheterization to the MHT or ILT is difficult, after weighing the complications of tumor resection against those of the para-para method.
Limitations
This study was conducted at a single institution, and the number of cases was small. In addition, several types of surgical instruments and embolic particles were used, and the procedure was not strictly standardized because it included 3 different methods of aspiration and removal of embolic particles. Furthermore, postoperative MR images were obtained after tumor removal in many cases, raising the issue of accuracy in assessing cerebral infarction after tumor embolization. A more accurate assessment of the efficacy and risk of complications of the para-para method would require the evaluation of a larger number of patients undergoing embolization using a uniform procedure. In addition, a comparison with patients who underwent tumor removal and did not undergo embolization would be warranted for future studies to determine the efficacy of tumor embolization.
CONCLUSIONS
Tumor embolization from the MHT and ILT remains challenging and should be performed in selected cases. However, the use of the para-para method is expected to expand the indications.
Acknowledgments
The authors would like to thank Editage (www.editage.com) for the English language editing.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 23, 2023.
- Accepted after revision January 13, 2024.
- © 2024 by American Journal of Neuroradiology