Abstract
BACKGROUND AND PURPOSE: The prodromal stage of Alzheimer’s disease presents an imperative intervention window. This work focuses on using brain age prediction models and biomarkers from FLAIR MR imaging to identify subjects who progress to Alzheimer’s disease (converting mild cognitive impairment) or those who remain stable (stable mild cognitive impairment).
MATERIALS AND METHODS: A machine learning model was trained to predict the age of normal control subjects on the basis of volume, intensity, and texture features from 3239 FLAIR MRI volumes. The brain age gap estimation (BrainAGE) was computed as the difference between the predicted and true age, and it was used as a biomarker for both cross-sectional and longitudinal analyses. Differences in biomarker means, slopes, and intercepts were investigated using ANOVA and Tukey post hoc test. Correlation analysis was performed between brain age gap estimation and established Alzheimer’s disease indicators.
RESULTS: The brain age prediction model showed accurate results (mean absolute error = 2.89 years) when testing on held out normal control data. The computed BrainAGE metric showed significant differences between the stable mild cognitive impairment and converting mild cognitive impairment groups in cross-sectional and longitudinal analyses, most notably showing significant differences up to 4 years before conversion to Alzheimer’s disease. A significant correlation was found between BrainAGE and previously established Alzheimer’s disease conversion biomarkers.
CONCLUSIONS: The BrainAGE metric can allow clinicians to consider a single explainable value that summarizes all the biomarkers because it considers many dimensions of disease and can determine whether the subject has normal aging patterns or if he or she is trending into a high-risk category using a single value.
ABBREVIATIONS:
- AD
- Alzheimer’s disease
- Aβ
- amyloid-β
- BrainAGE
- brain age gap estimation
- cMCI
- converting mild cognitive impairment
- NC
- normal control
- LVV
- lateral ventricular volume
- MAE
- mean absolute error
- MCI
- mild cognitive impairment
- MoCA
- Montreal Cognitive Assessment
- NABM
- normal-appearing brain matter
- p-tau
- phosphorylated-tau
- RFC
- Random Forest Classifier
- RFR
- random forest regressor
- sMCI
- stable mild cognitive impairment
- SVM
- support vector machine
Alzheimer’s disease (AD) causes accelerated loss of cognition and memory, and is the most common type of dementia.1,2 AD is expected to impact 1 in 85 persons worldwide within the next 30 years.3 Mild cognitive impairment (MCI) is the prodromal phase of AD, characterized by abnormal changes in cognitive domains that have not reached the severity of AD.4,5 This prodromal phase represents a window in which interventions can be applied to reduce the risk of progressing to AD. The MCI phase is important to target because >50% of individuals with MCI eventually progress to AD.6 Subjects with MCI who convert to AD are referred to as converting MCI (cMCI) as opposed to subjects with stable MCI (sMCI) who have cognitive issues but do not progress to AD. Distinguishing between subjects with cMCI and sMCI can aid in early disease detection, as well as patient stratification in large clinical trials. Incorrect patient grouping is one of the most common causes of failed AD treatment trials.7 Quantitative biomarkers from MR imaging can help improve diagnostic accuracy and increase cohort homogeneity in AD treatment trials.
Aging is a time-dependent process in which humans undergo functional decline due to an accumulation of cellular damage.8 When this process is accelerated due to AD, individuals have rapid cognitive decline and neurodegeneration. There is a growing interest in the community to measure this accelerated aging through brain age techniques. Brain age is the predicted chronological age of a subject estimated by using a machine learning model trained on normative data. This model is used to test disease data, and a large gap between the predicted age and true age is a sign of accelerated aging. Leveraging the benefits of machine learning can allow precision and preventative medicine when it comes to early AD diagnosis.
Previous brain age estimation techniques have used established T1-weighted MR imaging biomarkers and cognitive measures9 to categorize subjects into cognitive groups and identify subjects with cMCI.10
This work investigated brain age on the basis of FLAIR MR imaging biomarkers and a Random Forest Classifier (RFC) to differentiate between sMCI and cMCI in cross-sectional and longitudinal analyses. FLAIR MR imaging is routinely acquired in clinical settings, and FLAIR biomarkers have high translation potential. The FLAIR sequence highlights white matter disease and white matter lesions, which are related to many neurologic disorders,11⇓-13 cognitive impairment, age, and CSF biomarkers.11,14⇓⇓-17 Twelve FLAIR biomarkers related to intensity, texture, and volumes of objects in the brain were extracted using fully automated and validated algorithms and were used to train a machine learning algorithm to predict the brain age of subjects with cognitive impairment.
Four types of analysis were conducted. The first was a regression analysis to investigate the relationship between the predicted age and true age for each cognitive group. The second was a cross-sectional analysis, which compared the mean brain age gap estimate (BrainAGE) among the normal control (NC), sMCI, cMCI, and AD groups using ANOVA and the Tukey post hoc test. Longitudinal analysis was performed to compare regression slopes and intercepts of BrainAGE versus time among cognitive groups. Finally, the correlation between BrainAGE and CSF biomarkers and the APOE-4 genotype was investigated. The BrainAGE metric has benefits as a single, explainable biomarker that can be used for monitoring and diagnostic purposes or for generating homogeneous cohorts for clinical trials.
MATERIALS AND METHODS
Experimental Data
The Alzheimer’s Disease Neuroimaging Initiative, an international data set with longitudinal imaging for studying AD, was used throughout all analyses.18 A total of 3072 imaging volumes from 978 patients was used for cross-sectional and longitudinal analysis. The Montreal Cognitive Assessment (MoCA) score was used to label subjects as NC (MoCA > 26), MCI (19 < MoCA < 25), and AD (MoCA < 18). The MCI class is divided into sMCI and cMCI), depending on the analysis.
For cross-sectional analysis, a subject with MCI was labeled as cMCI if their MoCA score progressed from the MCI range to the AD range. The scan immediately prior (approximately 1 year) to AD conversion was labeled cMCI. Only the cMCI scan before conversion was retained for the analysis to avoid data leakage. If a subject remained within the MCI MoCA range for all time points, they were labeled sMCI. For longitudinal analysis, subjects labeled MCI who converted to AD in later scans were assigned a cMCI label for up to 6 years before AD conversion and were included to analyze disease progression across time. All scans after progressing to AD were considered AD. For subjects with cMCI, the longitudinal time points were normalized so that time point 5 is 1 year before converting to AD. This normalization was implemented to investigate how the subjects with cMCI progressed leading up to AD conversion. A summary of the sample sizes used for the cross-sectional and longitudinal analyses by the cognitive group is shown in Table 1.
FLAIR MR imaging data setsa
MR Imaging
FLAIR MR images were acquired from 58 centers worldwide on 3T machines from GE Healthcare, Siemens, and Philips Healthcare. Pixel spacing was 0.8594 mm, TR = 9000–11,000 ms, TE = 90–154 ms, TI = 2200–2500 ms.
Biomarker Measurement
FLAIR MR imaging volumes underwent bias field correction and intensity standardization to align intensity ranges of each tissue across data sets.19 Intracranial volume segmentation was performed using a convolutional neural network to extract brain tissue.20 Note that 31 volumes were excluded due to poor intracranial volume segmentations significantly impacting downstream biomarker extraction. Using the standardized imaging volumes, 6 volume, 3 texture, and 3 intensity biomarkers were extracted. Thresholding was used to extract total brain volume and CSF volume,19 while lateral ventricular volume (LVV) and white matter lesions were extracted using deep learning techniques.12,21 Subarachnoid CSF volume was computed as the difference between the CSF and LVV masks. The texture biomarkers, macrostructural damage, microstructural damage, and microstructural integrity were computed using spatial correlation and local binary patterns of intensities within regions of the normal-appearing brain matter (NABM), which is defined as the entire intracranial volume excluding the CSF and white matter lesion.14 Using NABM, we calculated the mean intensity, skewness, and kurtosis of the intensity distribution as the intensity features.
Brain Age Model
A brain age prediction model was developed to examine differences among NC, sMCI, cMCI, and AD using FLAIR biomarkers. Four different machine learning models were implemented, and their performance was compared using an RFC, logistic regression, support vector machine (SVM), and a random forest regressor (RFR). Note that for the discontinuous classifiers, the ages must be rounded to the nearest integer. A 70/30 (n = 1187 / n = 509) training/testing split was used. All normative (cross-sectional) data were used to train the model, which included FLAIR biomarkers and chronological age. Because all data are from a normative sample, the RFC is modeling healthy brain aging. A held out NC subset was used to verify model performance by measuring the mean absolute error (MAE) between the predicted brain age and ground truth chronological age. A common bias is frequently observed in brain age prediction in which models often overestimate the brain age of younger subjects, underestimate the brain age of older subjects, and predict subjects near the mean age more accurately.22⇓-24 Common practice is to apply the following statistical bias correction to account for this:
 While other methods have been attempted for bias correction of brain age, this technique has been found optimal.25 Note that the slope and intercept used in Equation 1 are from the true age versus predicted age of the NC group. Corrected brain age versus true age scatterplots and regression lines were analyzed using the Pearson correlation coefficient.
While other methods have been attempted for bias correction of brain age, this technique has been found optimal.25 Note that the slope and intercept used in Equation 1 are from the true age versus predicted age of the NC group. Corrected brain age versus true age scatterplots and regression lines were analyzed using the Pearson correlation coefficient.
BrainAGE Computation
By means of the optimal normative model, the remaining held out data that included subjects with sMCI (n = 1976), cMCI (n =68), and AD (n = 1707) were used to predict brain age. Franke et al26 showed how a relevant metric called BrainAGE, which is the difference between predicted brain age and true chronological age, can be used as a biomarker in many applications, including the assessment of neurologic, neuropsychiatric, and neurodegenerative diseases (Equation 2). The authors found a significant BrainAGE difference between NC/sMCI and cMCI/AD groups, demonstrating the viability of a BrainAGE biomarker.

Higher positive values indicate accelerated aging. The mean BrainAGE for each cognitive group was compared via ANOVA and Tukey post hoc analysis to investigate group differences.
Longitudinal BrainAGE
To analyze BrainAGE further, we used it as a biomarker to investigate disease progression across time for each disease group. The held out longitudinal data for this experiment included sMCI (n = 1976), cMCI (n = 161), and AD (n = 1707). Compared with the cross-sectional analysis which considers only the scan before conversion to AD as cMCI, in this analysis, the longitudinal labeling includes volumes from subjects who converted up to 6 years prior. The normative model was used to predict the brain age from each subject and time point. BrainAGE versus time regression lines were generated using the following equation: BrainAGE ∼ time + diagnosis + time + diagnosis. The slopes and intercepts of the regression lines for each diagnostic category were compared via ANOVA and Tukey post hoc. Cohen’s d was computed for the slopes and intercepts of the groups to determine the effect size. In order to determine how much time before conversion cMCI subjects show significant differences compared to sMCI subjects, t tests were performed comparing the BrainAGE of cMCI subjects from each time point with the entire sMCI group.
Biomarker Correlation
The BrainAGE metric and its relation to cognitive decline were further analyzed by correlation with phosphorylated-tau (p-tau) and amyloid-β (Aβ) CSF biomarkers and the APOE-4 genotype on cross-sectional data. These variables are known as criterion standard indicators of AD and cognitive impairment.27,28 Elevated p-tau and high levels of Aβ in the CSF are both strong indicators of AD. The presence of the APOE-4 allele is strongly associated with an elevated risk of developing AD.
RESULTS
All 12 FLAIR MR imaging biomarkers were extracted from the data set, and subjects were labeled as NC, sMCI, cMCI, or AD as in the subsection “Experimental Data.” Analyses were completed on an NVIDIA GeForce RTX 3090 Ti GPU with 32GB of RAM.
An RFC, SVM, logistic regression model, and an RFR were trained using all biomarkers from the NCs to create a brain age prediction model. On a held out NC test set, the MAE and correlation coefficient between the predicted brain age and the true age when using each model were reported (Table 2).
Brain age prediction model comparison based on MAE and correlation between true and predicted agea
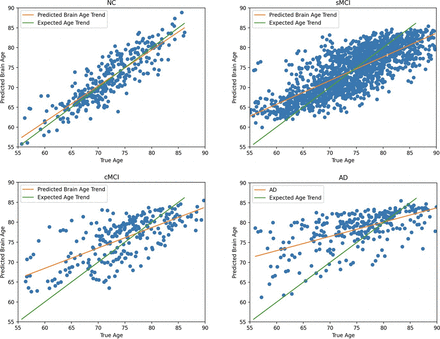
The results show the RFR is the optimal brain age prediction model because the model achieved an MAE of 2.46 years, which is among the lowest reported in the literature, which typically ranged from 4 to 9 years.9,29 The RFR model also yielded a strong correlation coefficient of 0.87 (P < .001) when comparing the predicted and true chronological ages of the NCs. The positive impact of the correction can be seen as the MAE and correlations improve for each of the 4 models after correction. Corrected predicted brain age versus true chronological age plots for NC, sMCI, cMCI, and AD when using the optimal model (RFR) are shown in Fig 1. True age–versus–predicted age plots before bias correction are shown in the Online Supplemental Data.
Corrected predicted brain age versus true age for NC, sMCI, cMCI, and AD using the RFR.
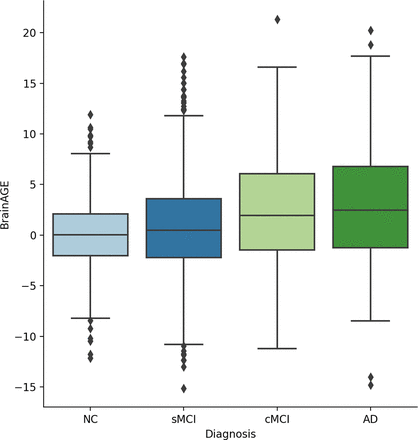
BrainAGE was calculated by using Equation 2, and the distributions for each cognitive group are shown in Fig 2. With a worsening cognitive state, the mean BrainAGE increases. Results from ANOVA and Tukey post hoc demonstrated that all comparisons had significantly different BrainAGE except for the NC and sMCI comparison (Table 3).
BrainAGE distributions.
ANOVA comparisons of BrainAGE
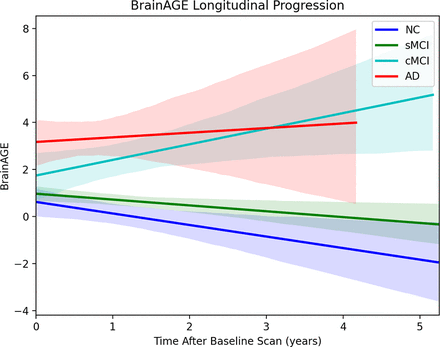
BrainAGE versus time from baseline for each cognitive group is shown in Fig 3. ANOVA and Tukey post hoc tests were performed to investigate differences between slopes and intercepts of the BrainAGE-versus-time regressions across all cognitive groups. The only significant differences (P < .05) in BrainAGE slopes were found between cMCI and NC and cMCI and sMCI. All intercept comparisons with AD showed significant differences (P < .01). All slope comparisons showed a large effect size and practical significance (d > 0.80) except for the NC/sMCI and cMCI/AD comparisons. All AD intercept comparisons showed large effect sizes (d > 0.80). ANOVA and Tukey’s post hoc results comparing slopes and intercepts are summarized in Table 4.
BrainAGE comparison across time after baseline scans for all cognitive groups.
ANOVA comparing slope and intercept of BrainAGE versus timea
Table 5 shows the t test results comparing the mean BrainAGE of the cMCI group at 1-year time intervals with the entire sMCI data set. There were significant BrainAGE differences (P < .05) between sMCI and cMCI starting at time interval 2–3, which indicate that the biomarkers can detect cMCI 3–4 years before AD conversion (time point 5 represents 1 year before AD conversion, time point 4 is 2 years before conversion, and so forth). Significant differences (P < .05) also exist in time 3–4 and time 4–5 intervals for the sMCI-cMCI BrainAGE comparison. Correlation analysis was performed between the computed BrainAGE metric and the existing criterion standard AD conversion indicators p-tau and Aβ CSF biomarkers and the APOE-4 genotype. Significant correlation (P < .05) was found between each comparison. A complete summary of the results of each experiment is shown in Table 6.
T test comparing BrainAGE of subjects with cMCI at 1-year intervals with sMCI data seta
Results summary from the brain age prediction model
DISCUSSION
This is the first work that uses FLAIR MR imaging biomarkers to predict the brain age of cognitively impaired subjects because T1-weighted MR imaging has been most commonly used in the past.9,29 Twelve biomarkers related to volume, intensity, and texture were extracted using previously validated tools.12,19⇓-21 An RFC trained and validated on held out NC subjects was developed to distinguish between cognitive groups cross-sectionally and longitudinally.
The BrainAGE metric quantifies signs of accelerated aging using the 12 FLAIR-only biomarkers, which encapsulate various aging mechanisms. The volume biomarkers summarize global changes in volume as well as specific ROIs. The texture features measure the microstructural properties of the GM and WM, which are correlated with mean diffusivity and fractional anisotropy in DTI,14 while intensity features show signs of demyelination, edema, and ischemia.30 The BrainAGE metric reduces the dimensionality of multiple features and summarizes the pathologic processes into a single value that successfully quantifies the amount of accelerated aging. Combining 12 different biomarkers accounts for the intersubject heterogeneity of AD progression.
The brain age prediction model validation yielded an MAE of 2.46 years between the corrected brain age and true age on a held out NC set, which is the lowest found in the literature when testing a dementia cohort. This finding demonstrates that the proposed FLAIR biomarkers provide a more accurate model compared with traditional approaches based on T1-weighted MR imaging. This result may be attributed to the unique insights FLAIR MR imaging provides, such as the ability to highlight white matter disease or increase LVV segmentation accuracy due to increased CSF-to-tissue contrast. There were significant differences in BrainAGE in all cross-sectional comparisons except NC/sMCI. Most notably, BrainAGE showed significant differences between sMCI and cMCI groups, demonstrating that FLAIR BrainAGE can identify high-risk subjects with MCI.
In the longitudinal analysis, the BrainAGE slope of the cMCI group showed significant differences compared with the NC and sMCI groups and no significant differences with the intercepts of NCs and subjects with sMCI. Therefore, at baseline, subjects with cMCI have profiles similar those of NCs and those with sMCI, but they neurodegenerate at an accelerated rate. Franke et al26 demonstrated significant differences (P < .050) between the slope of the BrainAGE-versus-time regression of NC/sMCI (group 1) and cMCI/AD (group 2), whereas the proposed model shows significant differences between the sMCI and cMCI groups specifically. This model is more applicable clinically because distinguishing between “low-risk” subjects with MCI (sMCI) and “high-risk” subjects with MCI (cMCI) offers more value because appropriate intervention can prevent AD conversion. Interestingly, the cMCI group had a greater rate of change of BrainAGE in compared to the AD group. This further highlights the ability of this metric to identify high-risk subjects because they are progressing at a faster rate than subjects with AD.
These results coincide with findings in Bahsoun et al14 that the MCI cohort shows more rapid changes in some NABM texture and intensity features with age in compared to subjects with AD. While previous studies using T1-weighted MR imaging showed subjects with AD progressing at a faster rate than those with cMCI,26 it is hypothesized that the increased rate of change of cMCI BrainAGE could be attributed to the unique biomarkers from FLAIR MR imaging. Using FLAIR NABM features in addition to established volume-based biomarkers uncovered the trend that subjects with cMCI have a greater rate of change in BrainAGE with time in compared to those with AD. Contrasted to NCs, sMCI, and cMCI, subjects with AD had a significantly higher baseline disease (P < .05). The BrainAGE of the AD group did not show significant slope differences between NC and sMCI groups, but there were significant differences when comparing intercepts. This finding indicates that baseline disease is higher in subjects with AD but that degeneration is at a rate similar to that of NCs and subjects with sMCI. No significant differences were found in any NC/sMCI comparisons, demonstrating that subjects with sMCI have neurodegenerative profiles similar to those of NCs. BrainAGE can perform longitudinal monitoring using a single value that encapsulates various dimensions of disease (volume, intensity, and texture). Otherwise, each individual biomarker would have to be monitored individually across time, and disease heterogeneity among patients would make it difficult to identify high-risk subjects on the basis of 12 different values.
To determine how far in advance the FLAIR-based BrainAGE showed differences between subjects with sMCI and cMCI, t tests were performed at 1-year intervals. There were significant differences between the sMCI and cMCI groups up to 4 years before conversion to AD. The earlier high-risk subjects are identified, the sooner a patient-specific neuroprotective treatment plan can be implemented. Existing brain age prediction models were able to predict AD conversion with adequate accuracy within 12 months’ follow-up,9 but none reported the ability to distinguish between sMCI and cMCI in terms of years before conversion. Given the emergence of new AD therapies such as anti-amyloid antibodies31 and peptide-based biotherapeutics,32 using biomarkers to stratify patients into more homogeneous groups can help determine whom to treat on a preclinical level. On a clinical level, this stratification could lead to a reduction in the incidence of AD and an improvement in the quality of life of individuals with cognitive impairment.
Differences in BrainAGE between subjects with cMCI and sMCI, both cross-sectionally and longitudinally, demonstrate the value and potential of BrainAGE as a biomarker. To understand the relationship of the proposed BrainAGE biomarker to known pathologic mechanisms of AD, we correlated BrainAGE with p-tau and Aβ CSF biomarkers and the APOE-4 genotype. BrainAGE showed significant correlation (P < .05) with p-tau, Aβ, and APOE-4. AD is characterized by an accumulation of p-tau and Aβ in various cortical regions of the brain,33 while the APOE-4 protein helps transport different types of fat throughout the bloodstream. Recent studies have suggested that problems related to the ability of brain cells to process lipids may play a key role in the development of AD.34 The significant correlations of the BrainAGE metric with all 3 of these criterion standard AD indicators demonstrate its strength in identifying these high-risk subjects.
Time normalization of the cMCI group may be considered a limitation of the longitudinal analysis as it is needed to align and analyze the progression of high-risk subjects as they approach AD conversion. Furthermore, the proposed metric should be analyzed with respect to clinical variables such as sex, lifestyle, socioeconomic status, and ethnicity. Although sex variables were available, the small cMCI sample size for the cMCI group would make it difficult to analyze sex-specific trends. In the future, we will consider adding data sets to further investigate these trends.
CONCLUSIONS
The BrainAGE metric demonstrates the ability to distinguish between subjects with sMCI and cMCI both cross-sectionally and longitudinally. The training of the brain age prediction model was performed on a held out NC data set. Doing so allowed the classifier to successfully identify subtle deviations from normal aging on diseased test samples and yielded BrainAGE values that showed significant differences among cognitive groups, most importantly between subjects with sMCI and cMCI. The brain age prediction model offers the ability to easily quantify the extent of the atypical aging into a single-valued metric while providing another method to distinguish among cognitive groups cross-sectionally and monitor accelerated aging longitudinally to identify, up to 4 years before conversion, subjects with MCI who are at an increased risk of developing AD. It can be concluded that this single-value metric successfully summarized the neuroanatomic state of the subjects, thus offering a maximally explainable and interpretable measure for the cognitive state of subjects both cross-sectionally and longitudinally. The BrainAGE metric showing significant differences between sMCI and cMCI up to 4 years before AD conversion is a major finding of this work. It would provide clinicians with the ability to make earlier diagnoses, thus giving them more time to intervene and help prevent further neurodegeneration toward the permanent damage caused by AD, leading to improving the quality of life for aging individuals and a reduction in AD prevalence.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received May 26, 2023.
- Accepted after revision October 13, 2023.
- © 2023 by American Journal of Neuroradiology