Abstract
BACKGROUND AND PURPOSE: There is no clear association between plaque distribution and postoperative complications in patients with basilar artery atherosclerotic stenosis. The aim of this study was to determine whether plaque distribution and postoperative complications after endovascular treatment for basilar artery stenosis are related.
MATERIALS AND METHODS: Our study enrolled patients with severe basilar artery stenosis who were scanned with high-resolution MR imaging and followed by DSA before the intervention. According to high-resolution MR imaging, plaques can be classified as ventral, lateral, dorsal, or involved in 2 quadrants. Plaques affecting the proximal, distal, or junctional segments of the basilar artery were classified according to DSA. An experienced independent team assessed ischemic events after the intervention using MR imaging. Further analysis was conducted to determine the relationship between plaque distribution and postoperative complications.
RESULTS: A total of 140 eligible patients were included in the study, with a postoperative complication rate of 11.4%. These patients were an average age of 61.9 (SD, 7.7) years. Dorsal wall plaques accounted for 34.3% of all plaques, and plaques distal to the anterior-inferior cerebellar artery accounted for 60.7%. Postoperative complications of endovascular treatment were associated with plaques located at the lateral wall (OR = 4.00; 95% CI, 1.21–13.23; P = .023), junctional segment (OR = 8.75; 95% CI, 1.16–66.22; P = .036), and plaque burden (OR = 1.03; 95% CI, 1.01–1.06; P = .042).
CONCLUSIONS: Plaques with a large burden located at the junctional segment and lateral wall of the basilar artery may increase the likelihood of postoperative complications following endovascular therapy. A larger sample size is needed for future studies.
ABBREVIATIONS:
- HR-MR imaging
- high-resolution MR imaging
- LA
- lumen area
- MLN
- maximal lumen narrowing
- VA
- vessel area
- WA
- wall area
The basilar artery is the main artery for the posterior intracranial circulation, where atherosclerotic stenosis is frequently discovered in patients with ischemic events, such as stroke and TIA, which are responsible for 10.7% of strokes annually.1 Patients with symptomatic and severe basilar artery stenosis (≥70%) may benefit from endovascular treatment, including primary angiography, balloon-mounted stent placement, and self-expanding stent placement.2 Compared with the those in the anterior circulation, postoperative complications are higher in the posterior circulation with a reported risk of 21.6% in the basilar artery.3,4 Therefore, it is crucial to understand and prevent a high risk of complications occurring during endovascular treatment in patients with basilar artery stenosis.
The distribution of atherosclerotic plaques is associated with the risk of ischemic events.5⇓-7 For example, it is more likely that atherosclerotic plaques located near perforating orifices are symptomatic during stent placement and may have a “snow plowing” effect.8 Several postmortem studies have demonstrated that basilar artery perforators typically originate from the lateral and dorsal walls of the segment, distal from the anterior-inferior cerebellar artery, possibly explaining the high rate of postoperative complications.9,10 However, the relationship between the distribution of basilar artery plaque and postoperative complications has not yet been reported .
Understanding the distribution of basilar artery plaques can help in reducing the incidence of postoperative complications. Evidence from studies since the Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial has shown that the postoperative risk of endovascular treatment for intracranial stenosis can be reduced from 14.7% to 9.4% by excluding symptomatic patients with perforating infarction.11 The postoperative risk could be reduced excluding perforating infarction actually infers the plaque location, whether it is near perforating orifices, according to the phenotype of cerebral infarction. Presently, plaque distribution in axial and coronal positions can be precisely evaluated using high-resolution MR imaging (HR-MR imaging) and DSA.12,13 However, very few studies have explored the direct relationship between plaque distribution and postoperative complications after endovascular treatment from the axial and coronal positions for basilar artery stenosis. The current study investigated the distribution of basilar artery atherosclerotic plaques and their clinical relevance using HR-MR imaging and DSA. Our findings can help neurointerventionalists with better patient selection and, thus, lower the procedural risk.
MATERIALS AND METHODS
Subjects
This study was based on the Clinical Registration Trial of Intracranial Stenting for Patients with Symptomatic Intracranial Artery Stenosis (CRTICAS) data base (ClinicalTrials.gov identifier: NCT01994161).14 The protocol complies with ethics principles of the Declaration of Helsinki and good clinical practice and has been approved by the the review board and ethic committee of Xuanwu Hospital, Capital Medical University ([2013] 004). Written informed consent was obtained from all the patients. The results of this study have been reported in accordance with the Strengthening The Reporting Of Cohort Studies in Surgery (STROCSS) criteria.15
The study included patients with basilar artery stenosis who were enrolled between December 2013 and December 2015 in several high-volume tertiary centers in China. The inclusion criteria were as follows: 1) degree of stenosis of >70% as confirmed by DSA; 2) patients with basilar artery atherosclerotic stenosis who experienced ischemic symptoms, such as dizziness, vertigo, headache, double vision, slurred speech, and numbness or weakness; 3) treatment using endovascular therapy; 4) HR-MR imaging performed before the intervention; and 5) MR imaging performed before and within 72 hours after the intervention. The exclusion criteria were as follows: 1) stroke caused by occlusion of the basilar artery, 2) concurrent endovascular treatment of another intracranial or extracranial vessel, 3) basilar artery stenosis accompanied by moderate-to-severe vertebral artery stenosis, and 4) nonatherosclerotic causes (eg, Moyamoya disease, vasculitis, or dissection).
Imaging Protocols and Evaluation
Before the intervention, all eligible patients were examined using a 3T MR imaging scanner (Magnetom Spectra; Siemens) equipped with a standard 8-channel head coil. The scans were obtained using multiple sequences, including fast spin-echo T1-weighted imaging, TOF-MR angiography, and T1-weighted enhanced imaging. The parameters of the sequences are described in the Online Supplemental Data. HR-MR imaging was acquired in the sagittal plane covering the basilar artery vessel. Reconstruction of the axial, coronal, and sagittal views was required to analyze all the images. DSA examinations were also performed for all the eligible patients.
An image core lab (http://imagecorelabcn.com/) was used to review all the images. The clinical data of patients were undisclosed and not included in the statistical analyses. Before the formal assessment, 5% of data from the analyzed cohort was used to train raters. Whenever the agreement was excellent (reliability, >0.75) between 2 raters, the imaging data were formally evaluated. The maximal lumen narrowing (MLN) or reference site, lumen diameter, vessel area (VA), and lumen area (LA) at the MLN or reference site were manually evaluated. Reference sites were defined according to Warfarin versus Aspirin for Symptomatic Intracranial Disease (WASID) trial as normal segments proximal to the stenosis or distal vessels if the proximal segment was diseased.16
On the basis of HR-MR imaging and DSA, we observed the following vessel parameters:
Wall area (WA): difference between VA and LA (VA – LA).
Plaque burden: [(WAMLN – WAreference) / VAMLN] × 100%.
Remodeling index: VA MLN/VA reference.
Stenosis degree is (1-Luminal Diameter at MLN/Luminal Diameter at the Reference Site) × 100% from DSA.
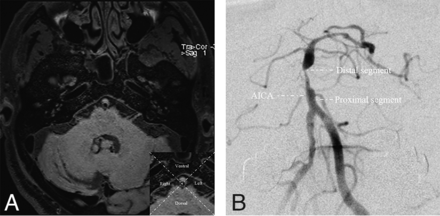
Using 2 perpendicular lines, we divided the cross-sectional plaque into 4 quadrants at the MLN: ventral, dorsal, left, and right.17 The left and right sites were classified as lateral sites. If the plaque was large and its thickest aspect spanned more than 2 quadrants, it was considered distributed between >2 quadrants (Fig 1A). In the coronal position, DSA was used to divide the basilar artery into 2 segments on the basis of the branches of the anterior-inferior cerebellar artery.18 Lesions crossing the anterior-inferior cerebellar artery were categorized as junctional segment lesions (Fig 1B).
A, The basilar artery is divided into 4 quadrants at the axial position based on high-resolution MR imaging. B, The basilar artery is divided into 2 segments referring to the AICA on the basis of DSA, the proximal and distal segments. The junctional segment is defined when the plaque involves both segments across the anterior-inferior cerebellar artery.
Interventional Procedure and Assessment of Outcome
All patients were treated by a team of neurosurgeons and neuroradiologists with extensive experience in endovascular treatment. Experienced operators determined the therapeutic strategy according to the lesion characteristics. Aspirin, 100 mg daily, was combined with clopidogrel, 75 mg daily for 5 days before, or a loading dose of aspirin and clopidogrel, 300 mg each, was used 1 day before endovascular treatment. Standard protocols for the procedure were followed as described previously.14 Three-month dual-antiplatelet therapy, comprising aspirin, 100 mg daily, and clopidogrel, 75 mg daily, was initiated following the intervention.
An experienced team of neurosurgeons and neuroradiologists investigated the postoperative ischemic events, including TIA and ischemic stroke. Ischemic stroke was defined as a neurologic deficit lasting >24 hours. TIA was defined as a neurologic deficit lasting <24 hours.19 Depending on whether postoperative outcome events occurred after endovascular treatment, patients were classified into an either ischemic or nonischemic events group.
Statistical Analysis
Data were analyzed using the SAS software (Version 9.4; SAS Institute). Quantitative variables are presented as means. Qualitative variables are presented as numbers and percentages. Descriptive analyses were performed for participants in the postoperative ischemic and nonischemic events group. The χ2 test or Fisher exact test was used to compare the categoric variables, as appropriate. The Student t test or Wilcoxon test was used to compare the quantitative variables. Univariate and multivariate regression analyses were performed to investigate the factors influencing the postoperative ischemic events. Variables with P < .1, along with sex, age, and treatment type, were included in the multivariate regression analysis. P < .05 was considered as statistically significant.
RESULTS
In total, 281 consecutive patients with severe symptomatic basilar artery stenosis underwent endovascular treatment. Among them, 140 patients having complete HR-MR imaging and DSA data were finally included in the analysis (Online Supplemental Data). The average age of these patients was 61.9 (SD, 7.7) years. Twenty-six (18.5%) patients underwent primary angioplasty, and 114 (81.5%) underwent stent placement, which included 30 balloon-mounted stents and 84 self-expansion stents. Furthermore, 16 patients with postoperative ischemic events, including 3 TIAs, 4 perforating infarctions, 7 artery-to-artery embolisms, and 2 mixed mechanisms were regarded as the ischemic events group, and 124 patients without postoperative ischemic events, as the nonischemic events group (Online Supplemental Data). Table 1 summarizes the clinical and lesion characteristics, with the exception of plaque burden (mean, 19.3% [SD, 18.3%] versus 3.7% [SD, 23.6%]; P = .047) between the ischemic events group and the nonischemic events group.
Clinical and lesion characteristics
A total of 140 basilar artery plaques were reviewed from the axial and coronal positions. In the axial view at the MLN site, 12.9% of plaques were distributed ventral to the basilar artery wall, and 22.1% in the lateral, 34.3% in the dorsal, and 30.7% in ≥2 quadrants. The plaques distributed in the lateral wall were more common in the ischemic events group than in the nonischemic events group (43.8% versus 19.3%, P = .049). In the coronal view, plaque distribution was most common at the segment distal to the anterior-inferior cerebellar artery (60.7%), followed by the segment proximal to the anterior-inferior cerebellar artery (35.0%), and least in the junctional segment (4.3%). Plaques distributed at the junctional segment in the ischemic events group were more frequent compared with the nonischemic events group (18.8% versus 2.4%, P = .020). The additional details of plaque distribution are presented in Table 2. The risk of ischemic events was the highest in patients with plaques located at the lateral wall (22.5%) and junctional segment (50.0%) of the basilar artery (Online Supplemental Data). Additionally, the high risk of ischemic events in patients with plaques located at the junctional segment of the basilar artery was associated with a large remodeling index compared with that in the nonischemic events group (mean, 1.38 [SD, 0.20] versus 0.82 [SD, 0.29]; 95% CI, 0.05–1.13; P = .049) (Online Supplemental Data).
Plaque distribution of the basilar artery
The multivariate logistic regression analysis showed that plaques distributed at the lateral wall (OR = 4.00; 95% CI, 1.21–13.23; P = .023) and junctional segments (OR = 8.75; 95% CI, 1.16–66.22; P = .036) as well as plaque burden (OR = 1.03; 95% CI, 1.01–1.06; P = .042) were associated with a higher risk of postoperative ischemic events (Table 3, Fig 2).
Relationship between plaque distribution and ischemic events by multivariate analysis
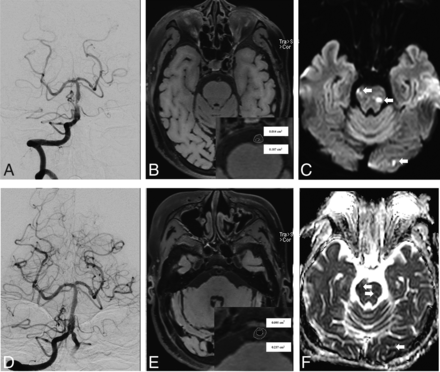
An adult patient presented with recurrent stroke for 1 month. A, Preoperative DSA revealed 79.3% stenosis at the distal segment of the basilar artery. The middle column shows cross-sectional T1-weighted basilar artery images at the MLN (B) and reference (REF) (E) sites. The VA and LA at the MLN (B, VA = 0.157 cm2, LA = 0.014 cm2) and REF (E, VA = 0.237 cm2, LA = 0.075 cm2) sites were manually traced for measuring after zooming in ×400. The plaque burden was calculated as 16.6% using [(WAMLN-WAREF) / VA MLN] × 100%. D, A 2.5 × 8 mm Apollo balloon-mounted stent (MicroPort NeuroTech) was positioned at the stenotic segment. DWI (C) and ADC imaging (F) confirmed a new mixed type of infarction (white arrows) after the intervention.
DISCUSSION
In this study, symptomatic patients with severe basilar artery stenosis were enrolled to investigate the correlation of atherosclerotic plaque distribution with postoperative complications. The risk of postoperative ischemic events in patients with severe symptomatic basilar artery stenosis was 11.4% (16/140). Although plaque distribution of the basilar artery was most commonly observed at the dorsal wall of the basilar artery in the axial (34.3%) and distal segments in the coronal view (60.7%), the risk of postoperative complications was highest when the plaques were situated in the lateral wall and junctional segment of the basilar artery. In addition to the large plaque burden, plaques located at the lateral wall and junctional segment of the basilar artery were independent risk factors for postoperative ischemic events.
The high risk of postoperative complications is a substantial limitation for endovascular treatment of intracranial atherosclerotic stenosis, especially in the posterior circulation.4,20 The basilar artery is the main artery for the posterior circulation, which has the highest risk of postoperative complications for endovascular treatment among the intracranial arteries.3 The posterior circulation appears to be more capable of plaque burden because it has a lower blood flow and less sympathetic innervation compared with the anterior circulation.21⇓⇓-24 Additionally, the number of perforating arteries in the basilar artery is significantly higher than that of the other intracranial arteries.25 These reasons explain the high risk associated with endovascular treatment. Previous studies have suggested that SAMMPRIS, strict patient selection, technical development, and incremental experience of the operators reduces the risk of postoperative complications for endovascular treatment.26 Our study showed a relatively lower risk compared with SAMMPRIS (21.6%) and is comparable with several studies (11.2%–14.2%).27,28 However, the prevention of postoperative complications is necessary to maximize the benefits of endovascular treatment.
It is highly likely that postoperative complications can be reduced by assessing the plaque distribution and features.18 Plaque distribution has been abundantly indicated to be a significant indicator of postoperative complications, thus revealing that postoperative complications of intracranial atherosclerotic plaques located in the posterior circulation were higher than those in the anterior circulation (12.1% versus 6.6, P < .01).29⇓-31 However, the association of local distribution of basilar artery atherosclerotic plaques with postoperative complications is unclear. Several researchers have hypothesized that plaques near the perforating orifices might be a determinant of postoperative complications through narrative analysis in plaque microanatomy studies.6,18,32,33
In this study, plaques present in the lateral wall were associated with a higher risk of postoperative complications.34 Penetrating arteries from the lateral and dorsal sides of the basilar artery have been observed in previous studies.10 According to the anatomic studies, 65.3% of penetrating arteries originate from the lateral side of the basilar artery, whereas 34.7% originate from the dorsal side.35 Additionally, the anastomosis rate of penetrating arteries emerging from the basilar artery is high (range, 41.6%–66.6%).9 From an axial perspective, the anastomosis rate of penetrations emerging from the dorsal side was higher than that on the lateral side.36 Moreover, 99% of the anastomoses of penetrating arteries from the dorsal side of basilar artery are located on the ventral surface of the pontocerebrum surface, 57.5% of the anastomoses occur between the penetrating arteries of the pontocerebrum, and 21.3%, between penetrations originating from the basilar artery and anterior-inferior cerebellar artery.36 As a result, plaques involving the lateral side of the basilar artery may be a risk factor for endovascular treatment for patients with severe basilar artery stenosis.
In addition, a large plaque burden is associated with postoperative complications, which may be due to a large plaque burden being more vulnerable during the expansion process of the balloon or stent.37 Furthermore, a large plaque burden has a high risk of initial and recurrent ischemic symptoms in patients with intracranial stenosis.38⇓-40 Thus, an evaluation of both plaque burden and distribution characteristics may be helpful in excluding high-risk patients from endovascular treatment of basilar artery stenosis.
This study has some limitations. First, although the study cohort had a larger sample size compared with other HR-MR imaging studies, the incidence of postoperative complications was low, comprising only 16 cases.17, 41 Therefore, the impact of plaque location on different stroke mechanisms (eg, artery-to-artery and perforating stroke) in symptomatic basilar artery stenosis is currently unavailable and warrants further analysis. Second, plaque characteristics from the axial position based on HR-MR imaging were assessed at the site of the MLN and not in other planes of plaque. The site of MLN was considered as the evaluation level because stress is most pronounced during endovascular therapy and may be associated with postoperative complications. Third, plaque burden was not analyzed separately at various plaque distributions from the axial and coronal positions to determine the potential mechanism of plaque distribution, which was constrained by the small number of cases of postoperative complications. This issue warrants further analysis with a larger sample size.
CONCLUSIONS
The current study showed that a large burden of plaques located at the lateral wall and junctional segment of the basilar artery may serve as predictive factors for postoperative complications during endovascular treatment. This finding may be helpful in selecting appropriate patients for endovascular treatment among symptomatic patients with basilar artery stenosis.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
This study was funded by the National Key Research and Development Program of China (2016YFC1301703), Beijing Science and Technology Planning Project (Z201100005520019), and Beijing Hospitals Authority's Ascent Plan (DFL20220702).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received November 20, 2022.
- Accepted after revision March 1, 2023.
- © 2023 by American Journal of Neuroradiology