Abstract
SUMMARY: With the routine use of high-resolution heavily T2-weighted sequences to evaluate patients with hearing deficits, new, subtle phenotypes of cochlear malformations are being discovered and an increasing number of genotype-phenotype correlations are being found through a reverse phenotype approach, which can help guide geneticists. In this brief report, we present subtle malformations of the apical turn of the cochlea related to 3 genetic mutations, emphasizing the importance of a careful assessment of the cochlear apex.
ABBREVIATIONS:
- BOR
- branchio-oto-renal syndrome
- DEGCAGS
- DEvelopmental delay with Gastrointestinal, CArdiovascular, Genitourinary, and Skeletal abnormalities
- IAC
- internal auditory canal
- SNHL
- sensorineural hearing loss
With the use of high-resolution sequences in MR imaging assessment of patients with congenital sensorineural hearing loss (SNHL), newer phenotypes of cochlear malformations have been described, including several types of cochlear hypoplasias.1,2 There is increasing recognition that specific radiologic appearances of the cochlea and temporal bone may be associated with specific syndromes or genetic mutations.3⇓⇓-6 Thus, an imaging phenotype can suggest an underlying genotype, contributing to clinical and genetic work-up of the patient.
The normal cochlea has an apical turn that is smooth in contour and evenly tubular throughout its length. The apex (following the basal and middle turns) typically spans 180°–270° around the center point, resulting in a cochlea with 2.5–2.75 turns total.7
Here, we describe subtle malformations of the apical aspect of the cochlea in 5 patients with 3 different genetic abnormalities and cochleovestibular symptoms; these apical malformations were chosen among the authors by consensus. The patients were selected from the Great Ormond Street Hospital database of dysplastic cochleae. We retrospectively reviewed the database for all cases that involved an anomaly in the apical turn, yielding these 5 cases. All these cases had genetic diagnoses available. We describe possible genetic mechanisms that may cause apical turn anomalies, and emphasize the importance of not overlooking such subtle cochlear abnormalities.
CASE SERIES
TKFC-Related Disorder
A 7-year-old patient known to have a biallelic mutation in the TKFC gene (Mendelian Inheritance in Man, 618805), with congenital cataracts, microophthalmia, and developmental delay, underwent MR imaging of the brain and inner ear/internal auditory canal (IAC) for balance difficulties and hypersensitivity to loud and sudden noises without hearing loss.
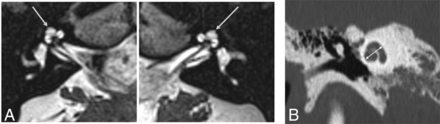
MR imaging of the brain demonstrated bilateral cataracts and cerebellar atrophy. A high-resolution 3D heavily T2-weighted sequence of the IAC revealed symmetric subtle cochlear abnormality characterized by a peculiar appearance of the upper part of the cochlea with an extra half-turn beyond the apical turn, resulting in >3 turns total (Fig 1A).7 The apical turn and the extra half-turn were not pointed but were smooth and flat like a normal apical turn. The cochlear height, measured in accordance with described methods in the literature (Fig 1B), was 6 mm (above the normal range of 4.3–5.4 mm).8,9 The cochleovestibular nerves were present and normal in course and caliber. The other inner ear structures were normal as well, including preserved internal partitioning of both cochleae.
A, High-resolution 3D heavily T2-weighted MR images of the inner ear and IAC in a patient with a TKFC-related disorder. There is an extra turn (arrows) at the apical portion of the cochlea beyond the usual apical turn and an overall greater cochlear height. The cochlear nerves are present, the modiolus is normal in appearance, and there is normal internal partitioning. Contrast this appearance with that of the normal cochlear apical turn seen in Fig 3B, without an extra turn beyond it. B, Coronal CT image through the cochlea shows the method to measure cochlear height as defined by Shim et al:8 The maximal height of the cochlea is measured along an axis perpendicular to the oval window (white line).
Presumed DEGCAGS Syndrome (ZNF699 Gene)
A 7-month-old boy with a homozygous variant of unknown significance in the ZNF699 gene (presumed diagnosis of DEvelopmental delay with Gastrointestinal, CArdiovascular, Genitourinary, and Skeletal abnormalities [DEGCAGS] syndrome [Mendelian Inheritance in Man, 619488]) presented with bilateral profound SNHL. He also had developmental delay, atrial septal defect, dysmorphic features, and congenital clavicle deformity.
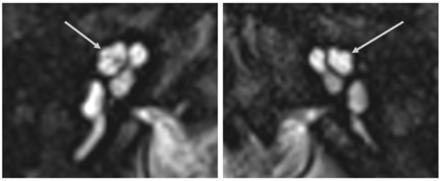
MR imaging of the IAC was performed to investigate the hearing loss. It demonstrated bilateral abnormality of the apical turn of the cochlea, very similar to the “thorny” cochlea described by Pao et al,10 in relation to SIX1 mutation in branchio-oto-renal syndrome (BOR). The cochlear apex was irregular, with a short, stumplike shape (Fig 2). There was mild hypoplasia of the cochlear nerve on the left, but otherwise the inner ear structures were normal bilaterally. The cochlear height was 5.8 and 5.7 mm on the right and left, respectively.
High-resolution 3D heavily T2-weighted MR images of the inner ear and IAC in a patient with a ZNF699 gene mutation. The uppermost turn of the cochlear apex following the middle turn has a short and stumpy shape (arrows).
SIX1-Related BOR
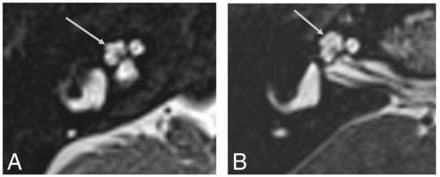
Searching our institutional archive for genetic malformations, we found, among patients with a genetic diagnosis of BOR syndrome, 3 patients with confirmed SIX1 mutation. Five of these 6 cochleae showed a characteristic “thorny” apical turn, with a small, pointed protuberant appearance (Fig 3). These patients presented with SNHL and other clinical abnormalities expected in BOR due to a SIX1 genetic mutation (ie, otic and branchial defects consistently seen, renal defects variably seen, and less prevalent than among individuals with BOR related to an EYA1 genetic mutation).11 Two of them were previously included in the study by Pao et al.10
High-resolution 3D heavily T2-weighted MR image of a typical SIX1-BOR protuberant “thorny” tip of the cochlea (arrow in A) in comparison with a healthy control in whom the apical turn is uniform and relatively flat and tubular (arrow in B).
DISCUSSION
All patients included in this case series presented with labyrinthine symptoms (4 with SNHL and 1 with balance difficulties and sound hypersensitivity) and very subtle malformations of the cochlear apical turn, which were overlooked by the reporting radiologists in 3 of the 5 cases.
The genetics of inner ear development are not completely understood, but we know that, at some point during embryologic development, genes polarize toward the cochlear (ventral) or vestibulocanalicular (dorsal) components of the otic capsule.12 It is also clear, from radiologic studies of cochleae fitting the description of the so-called cochlear hypoplasia type 4 anomaly, that the basal turn of the cochlea may be relatively preserved while the middle and apical turns are more affected.13 Indeed, this is the case in the “unwound” cochlea in patients with BOR14,15 and in the extreme hypoplastic cochlea in patients with Walker-Warburg syndrome.4
In patients with BOR, both EYA1 and SIX1 are expressed in the ventral (cochlear) part of the developing otic vesicle, but the expression of SIX1 is dependent on that of EYA1, and its expression is more prominent in the cochlear apex in murine models.10,16 This accounts for the different phenotypes seen among patients with BOR with mutations in the EYA1 versus the SIX1 gene, with only the apex being malformed in those with SIX1 mutations.
Although the role of the TKFC gene in ear development has not been reported, patients with this mutation may have progressive low-frequency hearing impairment.17 This specific type of hearing loss may be explained by morphologic abnormalities in the cochlear apex, which allows hearing of low pitches. In fact, the role of the shape of the apical cochlea has been correlated, in a comparative study among different species, with low-frequency hearing limits.18 Our case of TKFC-related disorder represents an example of clinicoradiologic correlation that can shed light on previously unknown functions of specific genes in ear development, including rare genetic mutations that lead to multisystemic abnormalities.
DEGCAGS syndrome is due to an autosomal recessive mutation in the ZNF699 gene and is characterized by neurodevelopmental delay, abnormal facial features, growth delay, syndactyly/polydactyly, and anemia/pancytopenia.19 This gene encodes for a nuclear zinc-finger protein with a possible function in nucleic acid binding.20 Little is known about the role of this gene, but patients described with this syndrome have SNHL as a constant symptom.19 It is, therefore, likely that the cause of the SNHL is related to a specific role of this gene in the development of the apical part of the cochlea. Again, the reverse phenotyping approach in this case can help define as yet suboptimally understood gene functions.
CONCLUSIONS
Subtle abnormalities of the inner ears, particularly those involving the apical part of the cochlea, can be easily overlooked, even with the use of optimal 3D high-resolution heavily-T2-weighted sequences on a 3T MR imaging scanner. We described 5 patients with 3 genetic abnormalities, all characterized by labyrinthine symptoms and malformation of the apical aspect of the cochlea and otherwise normal inner ear anatomy. These findings correlate with clinical symptoms of hearing impairment and shed light on the role of rare genetic abnormalities in inner ear development. A diligent search for these at-times subtle findings must be conducted, especially in cases of MR imaging of the IAC with seemingly negative findings in symptomatic patients.
This report adds to the growing evidence of genotype-phenotype correlation between syndromic and/or congenital deafness and anomaly of the cochlear apex in children.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received August 11, 2022.
- Accepted after revision November 15, 2022.
- © 2023 by American Journal of Neuroradiology