Abstract
BACKGROUND: Facial synkinesis, characterized by unintentional facial movements paired with intentional movements, is a debilitating sequela of Bell palsy.
PURPOSE: Our aim was to determine whether persistent peripheral nerve changes arising from Bell palsy result in persistent altered brain function in motor pathways in synkinesis.
DATA SOURCES: A literature search using terms related to facial paralysis, Bell palsy, synkinesis, and fMRI through May 2021 was conducted in MEDLINE and EMBASE. Additionally, an fMRI study examined lip and eyeblink movements in 2 groups: individuals who fully recovered following Bell palsy and individuals who developed synkinesis.
STUDY SELECTION: Task-based data of the whole brain that required lip movements in healthy controls were extracted from 7 publications. Three studies contributed similar whole-brain analyses in acute Bell palsy.
DATA ANALYSIS: The meta-analysis of fMRI in healthy control and Bell palsy groups determined common clusters of activation within each group using activation likelihood estimates. A separate fMRI study used multivariate general linear modeling to identify changes associated with synkinesis in smiling and blinking tasks.
DATA SYNTHESIS: A region of the precentral gyrus contralateral to the paretic side of the face was hypoactive in synkinesis during lip movements compared with controls. This region was centered in a cluster of activation identified in the meta-analysis of the healthy controls but absent from individuals with Bell palsy.
LIMITATIONS: The meta-analysis relied on a small set of studies. The small sample of subjects with synkinesis limited the power of the fMRI analysis.
CONCLUSIONS: Premotor pathways show persistent functional changes in synkinesis first identifiable in acute Bell palsy.
ABBREVIATIONS:
- ALE
- activation likelihood estimate
- BLINK
- eye blinking
- BP
- Bell palsy
- HC
- healthy controls
- PoC
- postcentral gyrus
- PrC
- precentral gyrus
- SMILE
- smiling
- REST
- rest blocks
Facial synkinesis is characterized by unintentional facial movements occurring simultaneously with intentional movements and develops weeks or months after facial nerve injury, most commonly following Bell palsy (BP). Periocular, midface, perioral, chin, and neck muscles can all be affected by synkinesis. Most commonly, facial synkinesis manifests as inadvertent lip movement during blinking or unintentional eye closure with smiling (Online Supplemental Data). The smile is frequently affected; patients often have uncoordinated activation of oral elevators and depressors, resulting in a lack of oral commissure elevation. The resulting asymmetric appearance of the face and uncoordinated facial movements impair facial expressions and conveyance of emotions, thereby negatively impacting a patient’s social life, work life, and self-image, which may lead to social isolation.1
Although the initial lesion in BP localizes outside the CNS, there is evidence that injury extends to the facial nucleus in the pontine brainstem, resulting in disorganized motor neuron axonal projections and loss of somatotopic organization.2,3 In animal models, injury of the facial nerve results in loss of somatotopic organization of the facial nucleus.2 The degree of somatotopic reorganization depends on the degree of injury and may contribute to abnormal recovery from BP and the development of synkinesis.4,5 Furthermore, synkinesis is thought to occur secondary to both aberrant peripheral nerve regeneration and neuronal reorganization in the facial nucleus following recovery from the original facial nerve injury.6 CNS alterations may, therefore, reflect compensatory mechanisms compounding the peripheral nerve abnormality that patients with synkinesis experience, but this idea has not yet been explored.
Although there are data indicating that CNS changes occur in patients with BP in the acute phase of illness,7-9 which may persist in some brain regions following recovery,9 there are scarce data on the changes in the brain due to synkinesis.10 In this study, we used fMRI during motor tasks that elicit facial synkinesis to characterize the brain changes associated with synkinesis. We first performed a meta-analysis of fMRI studies of facial movements in healthy controls (HC) and individuals scanned during acute BP to identify brain regions where there was convergent validation of brain activity specifically related to lip movements. Then, using fMRI to study facial movements in participants with synkinesis and those who had fully recovered following BP (control), we tested whether activation differences between the synkinesis and control groups converged on the regions identified in the meta-analysis. We hypothesized that synkinesis reflects persistent alterations in brain somatomotor pathways reported to be affected by BP; therefore, areas consistent with BP-related changes may also be affected in synkinesis.
MATERIALS AND METHODS
Systematic Review
A literature search using the Medical Subject Headings search terms, “facial paralysis,” “bells palsy,” “facial nerve disease,” “facial nerve paralysis,” “synkinesis,” “facial nerve,” “MR imaging,” “brain mapping,” “fmri,” “hemifacial,” was conducted from 1990 to May 5, 2020, to identify articles published in MEDLINE and EMBASE. Articles with only abstracts available, nonhuman studies, and non-English articles were excluded. Three independent investigators (N.A.K, M.L, and A.A.S.) reviewed the articles and collected data on standardized forms following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Inclusion criteria required studies that performed whole-brain analyses, used tasks requiring lip movement (smiling or lip pursing), and were contrasted to a resting condition; included participants with a history of BP and/or healthy individuals; and included BP participants imaged during the acute phase of illness. Studies were excluded if they were performed using a priori ROI-based analyses, rather than whole-brain analysis. While ROI-based studies are typically well-justified as a way to control statistical power and test specific hypotheses,9,11 they cannot be used in the meta-analysis because they introduce spatial biases associated with the selected brain regions.
Whole-Brain Meta-analysis
The meta-analysis of fMRI studies of facial movements in HC and BP groups was performed using the GingerALE software (Versions 3.0.2; http://www.brainmap.org).12-14 This approach estimates the above-chance probability of spatial clustering of activation loci from separate experiments compared with a random distribution across the spatial extent of the brain.13 The activation likelihood estimate (ALE) determines the spatial consistency across studies analyzed. The activation peaks of each study are modeled as spatial Gaussian distributions weighted by the sample size of the study. The distributions across studies estimate the modeled activation at each voxel.14 The resulting ALE is compared with a probability of a null distribution generated by a permutation test (1000 permutations).14 ALE maps are thresholded using a cluster-level family-wise error and a cluster-size threshold to reduce the probability of false-positive clusters.
Data in the included studies from the meta-analysis were converted into the Montreal Neurological Institute Colin27 template (http://neuro.debian.net/pkgs/mni-colin27-nifti.html) coordinate space. Data from BP groups were aligned so that the paretic side was on the left side of the body. For the studies that reported alignment of the paretic side of the face on the right, the left/right x-coordinates were flipped (positive-negative) for consistency.
Exploratory fMRI Study Methods
The study was approved by the Oregon Health & Science University institutional review board. Participants were identified for recruitment by searching the electronic medical record for International Classification of Diseases codes consistent with the diagnosis of BP.
Participants
Individuals were screened for eligibility on the basis of the following criteria: >1 year from the onset of BP and 18 years of age or older. Participants were excluded if pregnant, lacked decision-making capacity, were unable to safely undergo MR imaging, and/or had a history of viral skin lesions, epilepsy, dementia, brain tumors, multiple sclerosis, or stroke. Two groups of participants were enrolled: individuals with synkinesis following BP and a control group who fully recovered following BP.
All participants provided informed consent and underwent clinical assessment of their facial function, including photography/videography to capture the face at rest and during smiling and eye blinking. Photographs and videos were evaluated to assess facial function using the electronic clinician-graded facial function scale (eFACE; https://eface.ai/).15 eFACE provides reliable and reproducible measurements of facial function and disfigurement in those with facial paralysis.15 Additionally, participants completed the Synkinesis Assessment Questionnaire, a validated patient-graded instrument designed to assess facial synkinesis.16 Hand dominance was assessed by the Edinburgh Handedness Inventory.17
Image Acquisition
Scanning took place at OHSU’s Advanced Imaging Research Center using a 3T Magnetom Prisma whole-body scanner (Siemens), fitted with a 32-channel head coil. A T1-weighted MPRAGE anatomic scan was acquired with the following parameters: TR/TE = 2.4 sec/2.22 ms, TI = 1.0 sec, flip angle = 8°, matrix = 320 × 300, FOV = 320 × 300, section orientation = sagittal, voxel size = 1.0 × 1.0 × 0.8 mm. fMRI data consisted of 4–6 EPI blood oxygen level–dependent scans. Scans were acquired with the following parameters: TR/TE = 2000/30 ms, flip angle = 90°, slices = 35, in-plane resolution = 2 × 2 mm, section thickness = 2 mm, volumes = 140, acquisition time = 4 minutes 46 seconds.
Participants practiced and then performed a series of motor tasks during fMRI acquisition. Each scan contained alternating blocks of bilateral eye blinking (BLINK) and smiling (SMILE) separated by rest blocks (REST). These specific movements were chosen given the high likelihood of involvement of the smile and/or blepharospasm in facial synkinesis. Each block was 16 seconds long and repeated 5 times during each scan. A recorded message presented through MR imaging–compatible in-ear speakers (http://www.sensimetrics.com, model S-15) spoke “ready” 2 seconds before the onset of each block and then repeated the gesture to be executed (SMILE or BLINK) at 0.5 Hz during the task blocks to maintain a similar cadence between all participants. A “rest” command was presented at the beginning of the REST block.
The fMRI data were preprocessed and analyzed using the Analysis of Functional Neuro Images (AFNI) software suite (https://afni.nimh.nih.gov/). Slices from each volume were temporally aligned to account for differences in section time acquisition, motion corrected by realigning each volume to the minimum outlier volume from each fMRI time-series, and then aligned to the high-resolution anatomic volume. All runs were concatenated and voxel intensities were normalized and spatially smoothed with a 5.0-mm Gaussian filter. The 6 motion estimates and their derivatives were entered as nuisance regressors into the model. Volume-to-volume displacements of >0.3 mm in each time-series were censored in the regression model. The “ready” and “REST” signals were included as nuisance variables. The regressors for SMILE and BLINK were separately modelled with a canonical hemodynamic response function convolved with the task blocks. The data were aligned to the Montreal Neurological Institute 152_2009c template using linear and nonlinear warps to permit group-level data analysis. Synkinesis data were aligned with the synkinetic side on the left of the body.
Statistical analyses of the fMRI data were first performed at the subject-level and then entered into a group-level analysis using a linear mixed-effects analysis with 1 between-groups (control, synkinesis) factor and 1 (SMILE, BLINK) within-groups factor.18 A spatial threshold filter of 100 contiguous voxels was applied, and the family-wise error significance threshold was set to P < .005.
RESULTS
Systematic Review and Whole-Brain Meta-analysis
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, 14 articles were identified for full review (Online Supplemental Data). For the final meta-analysis, task-based data involving smiling were extracted from 7 publications, of which 5 studies included HC groups (Buendia et al;19 Calistri et al;20 Hesselmann et al;21 Song et al;11 Wang et al22) with a total combined sample size of 103 participants and 33 separate foci reported. Three studies contributed whole-brain analysis of acute BP participants (Calistri et al;20 Klingner et al;23 Klingner et al24) with a total combined sample of 78 BP participants and 60 foci reported across paretic and nonparetic sides (Online Supplemental Data).
The meta-analyses for the HC and BP studies were performed separately. In the HC group analysis, we identified 4 major clusters associated with lip movements (Fig 1 and Online Supplemental Data): 1) the left hemisphere comprising foci in the precentral gyrus (PrC) and postcentral gyrus (PoC); 2) along the medial wall of the prefrontal cortex; and 3) the left hemisphere, analogous to the first cluster, including the PrC and PoC; and 4) the right cerebellum.
ALE of the foci for HC studies (blue) from the 5 separate studies (n = 103) and BP studies (red) from 3 separate studies (n = 78). Yellow arrows indicate the location of significant ALE in the cerebellum of the HC group. Yellow chevrons indicate significant ALE in the left precentral gyrus, exclusively in the HC analysis. The lower row shows the same results on glass brains. Numbers indicate Montreal Neurological Institute z-coordinates. Left (L) is on left. R indicates right.
The BP meta-analysis showed substantial overlap with the HC group in the left hemisphere (Fig 1 and Online Supplemental Data) ipsilateral to the paretic side, including the PrC and extending anteriorly into the middle frontal gyrus. The second cluster was detected along the medial wall of the prefrontal cortex, similar to the findings in the HC studies. A third cluster lay anterior and inferior to the second cluster in the anterior cingulate gyrus.
A random-effects conjunction analysis of ALE maps14 from the HC and BP groups indicated consistency between the groups along the medial wall of the frontal lobe (Online Supplemental Data). A second volume of conjunction appeared in the left hemisphere at the junction of the PrC and PoC (Online Supplemental Data). A contrast analysis performed to identify differences between the groups detected no significant differences between clusters. Nevertheless, we noted in the BP group analysis no detection of a cluster in the right PrC and PoC areas, as was observed in the HC analysis.
Results of the fMRI Study
A total of 14 subjects, all right-handed, were included in the study; 7 subjects (1 woman) in the control group (61 [SD, 12] years of age) and 7 subjects (6 women) in the synkinesis group (60 [SD, 15] years of age). The control mean Synkinesis Assessment Questionnaire was 23.2 (SD, 4.77), compared with 73.3 (SD, 20.16) (P < .01) in synkinesis, consistent with facial synkinesis. The mean eFACE synkinesis score was 99.0 (SD, 1.91) in the control group and 70.6 (SD, 16.1) in the synkinesis group (P < .01), while the mean eFACE dynamic score was 97.9 (SD, 1.78) in the control group and 77.0 (16.1) in the synkinesis group (P = .02), confirming synkinesis and asymmetry with movement in the synkinesis group.
Using fMRI, the SMILE task produced greater signal change than BLINK bilaterally in the somatomotor areas, with activation foci centered on the central sulcus and the BLINK task producing greater signal change in the medial occipital cortex in areas corresponding to primary and secondary visual areas (Online Supplemental Data). The control group demonstrated greater activation across both SMILE and BLINK tasks in the medial supplementary motor cortex (Online Supplemental Data).
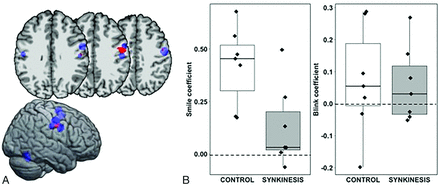
A single cluster associated with a group by task interaction was detected in the right PrC extending into the central sulcus, contralateral to the synkinetic side of the face (Fig 2). The interaction effect was examined by extracting β coefficients from the right PrC cluster for each subject, revealing that the synkinesis group had significantly lower signal in the SMILE condition compared with controls (Wilcoxon sum rank test: W = 42, P = .026) but did not differ under the BLINK condition (W = 28, P = .710) (Fig 2). No clusters associated with the BLINK task differed between the 2 groups.
A, Significant group (control, synkinesis) by condition (SMILE, BLINK) interaction (P < .005) (red). The ROI falls in the precentral gyrus and central sulcus in the right hemisphere contralateral to the synkinetic side of the face (Montreal Neurological Institute coordinates: x = 55, y = 7, z = 35). HC clusters from the meta-analysis are shown for reference (blue). B, Boxplots of the regression coefficients for the SMILE condition and BLINK condition by group.
DISCUSSION
Facial paralysis and synkinesis following BP alter the functional brain response in similar somatomotor cortical areas. A meta-analysis to identify common areas of activation by lip movements in healthy individuals and individuals with acute BP revealed that the PrC area active in HC studies was absent contralateral to the paretic side of the face in BP. In the fMRI study of synkinesis, the same PrC location contralateral to the synkinetic side of the face had significantly decreased signal, but only during the SMILE task (Online Supplemental Data and Figs 1 and 2). This finding in synkinesis suggests that that PrC changes are linked with aberrant nerve fiber regeneration and facial nucleus changes thought to be responsible for disorganized facial movements.6 Together with the meta-analysis of BP, the data suggest that synkinesis may reflect chronic changes in the PrC associated with the early effects of acute BP shown in prior studies, while hypoactivity in the Supplementary Motor Area (SMA) may reflect generalized chronic changes.
Previous fMRI studies of acute BP using both whole-brain and ROI analyses characterized changes in the brain during mouth movements as decreased signal in the somatomotor areas contralateral to the paretic side of the face.7,23 Smit et al9 demonstrated that as recovery progressed following BP, activation increased in the somatomotor region contralateral to the affected side of the face. However, in synkinesis, the hypoactivity in the PrC appeared to persist (Fig 2 and Online Supplemental Data). Changes in the CNS arising from peripheral nerve damage have been documented in several sensory pathways; for example, phantom limb sensations in upper limb amputation alter contralateral somatomotor areas25 and tinnitus affects the organization of auditory cortical fields.26 In these cases, there is evidence of remodeling within somatotopic sensory areas27 and in auditory cortical and subcortical pathways,28 respectively. A study of the effects of movement restriction for 2 weeks using casting of a healthy arm led to decreased connectivity between the ipsilateral and contralateral somatomotor areas, which returned to normal following removal of the cast.29 Similarly, there is evidence to support CNS involvement and cortical changes in BP, a disorder secondary to pure peripheral motor deafferentation without an effect on sensory afferents7-9,11,23 and the restoration of signal following recovery from BP.9 The results of the meta-analysis and synkinesis study indicate that the contralateral PrC in BP and synkinesis may be a common node; how the changes seen in the PrC during acute BP are linked to the aberrant innervation of facial musculature in synkinesis remains unclear. The PrC sits at the apex of the motor system, and its hypoactivity may reflect functional changes at multiple levels of the motor pathway.9,22,24
The study has several limitations. While there appears to be continuity between changes observed in the PrC from the BP meta-analysis and the fMRI study evaluating synkinesis, how this hypoactivity relates to peripheral changes and synkinesis is unknown. Additionally, the meta-analysis relied on a small set of studies that qualified for the analysis. Therefore, the relatively weak power of the analysis likely underestimated the brain locations affected by acute BP. The failure to detect significant activation clusters that differed between the BP and HC groups was also likely a reflection of the small sample sizes of the studies. The fMRI study also relied on a small sample and had limited statistical power. Consequently, changes in other cortical and subcortical areas may not have been detected. Additionally, the task design of simple lip movements and blinking provided a test of changes in hemodynamic function linked to these facial gestures and was consistent with prior studies of BP, providing a first step in examining the effects of synkinesis.
CONCLUSIONS
Premotor pathways show persistent functional changes in synkinesis, some of which are first identifiable in acute BP. The changes observed in the brains of participants with synkinesis likely reflect a confluence of neurophysiologic and behavioral adaptations to synkinesis. Understanding the CNS response to peripheral nerve injury and its sequelae can lead to improved clinical practices that enhance adaptation.
Footnotes
This work was supported by the OHSU Shared Resources Core Pilot Program, and by NIH S10OD021701 and NIH S10OD018224 for imaging and computing resources in OHSU’s Advanced Imaging Research Center.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received January 24, 2022.
- Accepted after revision June 28, 2022.
- © 2022 by American Journal of Neuroradiology