Abstract
BACKGROUND AND PURPOSE: Percutaneous sacroplasty is a variation of percutaneous vertebroplasty that has gained attention as a therapeutic option for patients with painful sacral insufficiency fractures due to osteoporosis or metastases. Additionally, percutaneous sacroplasty can also be used to treat painful sacral metastases without a pathologic fracture. The purpose of this retrospective study was to compare the efficacy and safety of fluoroscopy-guided percutaneous sacroplasty alone versus percutaneous sacroplasty plus radiofrequency ablation for the treatment of painful sacral metastases.
MATERIALS AND METHODS: For this retrospective study, 126 patients (with a total of 162 painful sacral metastases) were enrolled from October 2012 to February 2021 and assigned to receive either percutaneous sacroplasty plus radiofrequency ablation (n = 51, group A) or percutaneous sacroplasty alone (n = 75, group B). Four different approaches were used for percutaneous sacroplasty: transiliac, interpedicular, anterior-oblique, and posterior. The Visual Analog Scale, Oswestry Disability Index, and Karnofsky Performance Scale were used to evaluate outcomes.
RESULTS: The Visual Analog Scale, Oswestry Disability Index, and Karnofsky Performance Scale scores showed significant improvement in both groups after treatment (P < .05). The overall pain relief rate was significantly better in group A than in group B (90% versus 76%, P = .032). There were no significant differences in the incidence of polymethylmethacrylate leakage between the 2 groups or among the 4 different approaches (P > .05).
CONCLUSIONS: Both percutaneous sacroplasty alone and the combination of percutaneous sacroplasty and radiofrequency ablation are safe and effective for treatment of painful sacral metastases. The combination of percutaneous sacroplasty and radiofrequency ablation appears to be more effective than percutaneous sacroplasty alone.
ABBREVIATIONS:
- KPS
- Karnofsky Performance Scale
- ODI
- Oswestry Disability Index
- PMMA
- polymethylmethacrylate
- PSP
- percutaneous sacroplasty
- RFA
- radiofrequency ablation
- VAS
- Visual Analog Scale
Bone metastasis is common in advanced cancers and is often the cause of severe pain. It is estimated that ∼45% of patients with bone metastases do not receive adequate and appropriate treatment and therefore have avoidable pain.1 Metastasis in the sacrum can cause intractable pain and dysfunction, confine the patient to bed, and seriously impair his or her quality of life. The current standard of treatment for sacral metastases includes systemic and local therapies. Conservative treatment with bed rest, analgesics, chemotherapy, and bisphosphonates2 and radiation therapy—which are often used as first-line treatments—do not provide mechanical support or complete pain relief.3 Meanwhile, surgical treatment is limited by the prolonged recovery time and the high risk of complications and mortality.4
Percutaneous sacroplasty (PSP), developed from percutaneous vertebroplasty, is a promising option for the treatment of osteoporotic sacral insufficiency fractures and sacral metastases.5⇓⇓-8 However, the few studies to date on the use of PSP for the treatment of painful sacral metastases have had small sample sizes and/or did not examine the use of PSP in combination with other therapies.9⇓⇓-12
The aim of this retrospective study was to assess the safety and efficacy of PSP under fluoroscopy for the treatment of painful sacral metastases unresponsive to conservative treatments and to compare long-term outcomes after treatment with PSP alone versus PSP plus radiofrequency ablation (RFA).
MATERIALS AND METHODS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Patients
The institutional ethics committee of our hospital approved this study. Informed consent was obtained from all individual participants included in the study. Consecutive patients presenting with sacral metastases and severe pain at our clinic between October 2012 and February 2021 were approached for enrollment in this study. Those who expressed a willingness to participate were assigned to receive either PSP plus RFA or PSP alone. The inclusion criteria were the following: 1) older than 18 years of age; 2) sacral metastases with severe pain and an inability to walk or sit; 3) metastasis of ≤3 cm in diameter; 4) no relief with conventional treatments (opioids, radiation therapy, and chemotherapy); 5) unwilling to undergo or unfit for surgical treatment due to poor performance status; 6) life expectancy ≥3 months; and 7) willingness to provide a signed consent. The exclusion criteria were the following: 1) erosion of the neuroforamina or epidural tumor; 2) systemic infection; 3) uncorrectable coagulation disorder (international normalized ratio > 1.50, platelet count < 90 × 109/L); 4) allergy to polymethylmethacrylate (PMMA); or 5) concurrent severe cardiopulmonary disease. Before 2017, only PSP was performed in these patients. Since 2018, with the introduction of radiofrequency equipment, we have adopted a combination of PSP and RFA to treat these patients. Of the 126 patients who met the eligibility criteria, 51 were assigned to receive PSP plus RFA (group A), and 75, to receive PSP alone (group B). The ethics committee of our hospital approved this study. Informed consent was obtained from all participants.
Lesions
Although sacral metastases in the present study were not associated with pathologic fractures, these lesion zones were classified according to the Denis fractured zones.13 Among the 126 patients, there were 95 zone 1 metastases (ie, metastasis of the sacral ala lateral to the neural foramina); 30 zone 2 metastases (ie, metastasis in foramina area); and 37 zone 3 metastases (ie, metastasis involving the sacral body and spinal canal). The primary cancers included lung (n = 44), liver (n = 22), thyroid (n = 21), breast (n = 16), prostate (n = 10), kidney (n = 8), epithelioid hemangiosarcoma (n = 2), gallbladder (n = 1), colon (n = 1), and parotid (n = 1). Among the 126 patients with sacral metastases, 76 also received treatment for lesions at other locations, ie, the spine (56 patients) and the pelvis (20 patients). The PSP/RFA procedures were performed after radiation therapy in 17 group A patients and 29 group B patients. Radiation therapy was not indicated in 34 group A patients and 46 group B patients before the PSP/RFA procedures. All patients routinely received systemic medical therapy (including bisphosphonate). The Table shows the patients’ baseline characteristics and a summary of the results.
Baseline characteristics and clinical outcomes in patients with sacral metastatic tumors between the 2 groups
Procedure
Three doctors, all with >15 years of extensive experience in musculoskeletal intervention, performed PSP/RFA. The patient was placed on the operating table in the prone position. All procedures were performed with the patient under local anesthesia (2% lidocaine) and conscious sedation. Strict aseptic techniques were followed. The approach chosen (posterior, anterior-oblique, interpedicular, and transiliac) depended on the location of the lesion, with the aim being to reach the targeted lesion via the shortest distance without causing vessel or nerve damage (Figs 1 and 2). Generally, the anterior-oblique, transiliac, or posterior approaches were selected for lesions in the sacral ala, while the posterior approach was selected for lesions in other parts of the sacrum; the interpedicular approach was used for metastases in the sacral bodies.
Drawings show the puncture needles in place for the 4 different approaches: the transiliac approach (A), short-axis technique of posterior approach (B), long-axis technique of posterior approach (C), anterior-oblique approach (D), and interpedicular approach (E).
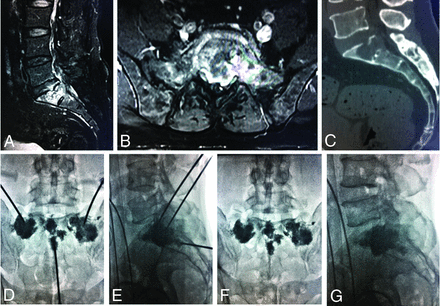
PSP procedure. A–C, Metastatic lesions in the sacral ala and bodies can be seen on enhanced sagittal and axial T1-weighted MR imaging and sagittal reconstruction CT images. D and E, Radiographs obtained during percutaneous cement injection with the patient in the supine position. F and G, After PSP, there is homogeneous and sufficient distribution of bone cement in the lesions.
The bone puncture needle—either a 13.8-cm 15-ga C1616A Bard TruGuide Disposable Coaxial Biopsy Needle (Bard Peripheral Vascular) or a 10-cm 13-ga beveled Murphy I bone puncture needle (Cook)—was inserted into the lesion under fluoroscopic guidance. Lateral and anterior-posterior fluoroscopic guidance was used to ensure a safe distance between the needle tip and critical structures such as sacral foramina and anterior surface of the sacrum. When necessary, the needle tip was rotated so that the beveled side faced the structure to be protected. In some cases, >1 needle was used. After the needle tip had entered the target lesion, PMMA (Palacos V; Heraeus Medical) was injected under continuous bilateral fluoroscopic monitoring. The injection was stopped when the bone cement reached the posterior wall of the sacrum, the edge of a sacral foramina, or the margin of the sacroiliac joint.
For patients receiving PSP plus RFA, the RFA was performed first. After installation of the bone needle, an RFA electrode with a 1- to 3-cm active tip (UniBlate 17-ga; AngioDynamics) was inserted coaxially into the needle, and ablation was applied with a power setting of 30 W. The ablation range was determined by lesion size, which was assessed by enhancement MR imaging and CT beforehand. The temperature at the tip of the needle ranged from 70°C to 90°C. Complete ablation was defined either by reaching a mean target temperature of 85°C maintained for at least 10 minutes or by reaching an obvious increase of impedance (so-called “roll-off”) twice, corresponding to a complete coagulation necrosis. In addition, neural reflex including acute severe sacral pain and paresthesia and/or dyspraxia in the levator ani or urethral sphincter of any patient was closely observed, and RFA was immediately stopped if the patient developed neurologic symptoms. After RFA, PMMA cement was carefully injected into the lesion. CT was performed immediately after the procedure to evaluate the cement distribution and identify leakage.
Outcome Evaluation
Two of the authors performed thorough clinical examinations of all patients before treatment, at 1 day after treatment, and then at 1, 3, 6, and 12 months after treatment. Outcome measures were technical success, complications, and improvement of pain and mobility. Technical success was defined as successful performance of puncture, RFA, and cement injection without major complications. The volume of each lesion was measured on the CT scans.14 The degree of cement filling was calculated by the maximum diameter of the cement deposit compared with the maximum diameter of the lesion.11 Clinical efficacy was assessed by measuring improvement in pain and function. Pain relief was measured by the changes in analgesic use, analgesic scale, Visual Analog Scale (VAS) score, and the overall rate of pain relief.5,15,16 Functional outcome was assessed using the Oswestry Disability Index (ODI) and Karnofsky Performance Scale (KPS).17,18 Major and minor complications were defined according to the reporting standards of the Society of Interventional Radiology.19 Major complications were those resulting in persistent morbidity or requiring an operative intervention, and minor complications were those that were transient, requiring only a temporary medical intervention.
Statistical Analysis
SPSS Statistics 22 (IBM) was used for statistical analysis. Normally distributed continuous variables were summarized as mean (SD) and compared between groups using the independent samples t test. Categoric variables were summarized as percentages and compared using the χ2 test. Statistical significance was at P < .05.
RESULTS
The 126 patients (69 men and 57 women) in this study had a mean age of 58.35 (SD, 13.62 ) years (age range, 18–89 years). A total of 162 lesions were treated in these 126 patients: 58 lesions in the 51 group A patients and 104 lesions in the 75 group B patients. The mean volumes of lesions were 7.22 (SD, 1.74) mL and 6.69 (SD, 1.96) mL in groups A and B, respectively (P >.05). Some adjacent lesions were treated through a single bone puncture needle. The procedures were well-tolerated by all patients, and the technical success rate was 100%. In the present study, 147 approaches were used for 162 lesions in total. The posterior approach was used in 60 patients; the transiliac approach, in 16 patients; the anterior-oblique approach, in 32 patients; and the interpedicular approach, in 39 patients. The mean procedural time was 45.5 (SD, 4.3) minutes (range, 37–49 minutes). The number of needles used per lesion ranged from 1 to 3 (mean, 1.38 [SD, 0.78]). The mean amount of cement injected per lesion was 6.42 (SD, 1.73) mL; range, 3–8 mL in group A versus 5.31 (SD, 1.52) mL; range, 3–8 mL in group B (P < .001). The mean degree of cement filling was 84.82% (SD, 0.37%) in group A, while it was 76.16% (SD, 0.77%) in group B (P < .05). In group A, the mean number of overlapping RFAs and complete ablation cycles was 1.89 (SD, 0.75) and 2.03 (SD, 1.54), respectively. The mean time taken for RFA was 7.54 (SD, 2.62) minutes.
No major complications occurred in group A; and only 1 major complication of extravasated cement requiring surgical intervention, in group B. Cement leakage occurred in 10/58 (17%) of the treated lesions in group A and 20/104 (19%) of treated lesions in group B. No group A patient had symptoms. However, 3 group B patients had symptoms: One had radicular pain, and 2 had of focal stabbing pain, which was due to soft-tissue cement extravasation; one of these patients needed surgical resection of the extravasated cement for relief of pain.
The mean hospital stay was 4.51 (SD, 1.69) days (range, 2–7 days), and mean postprocedural follow-up was 10.13 (SD, 6.78) months (range, 3–12 months). In group A, the mean VAS score decreased from 7.43 (SD, 1.56) before the procedure to 2.25 (SD, 1.35) on day 1 after the procedure (P < .001). On day 1 after the procedure, 46 patients reported pain relief, but 5 patients had no improvement. In group B, the mean VAS score decreased from 7.45 (SD, 1.61) before the procedure to 3.02 (SD, 1.55) on day 1 after the procedure (P < .001) (Online Supplemental Data). While 57 had pain relief, 19 had no improvement. The overall pain relief rate was significantly better in group A than in group B (90% versus 76%, P = .032).
On day 1 after the procedure, 46/51 (90.19%) patients in group A discontinued or reduced the dosage of narcotic analgesics. Postprocedural pain was controlled with weak opioid analgesics (4 patients) or nonsteroidal anti-inflammatory drugs (7 patients); the remaining 35 patients did not require any analgesic therapy after the procedure. The mean analgesic scale decreased from 3.28 (SD, 1.42) before the procedure to 0.93 (SD, 1.38) postprocedure (P < .001). In group B, 57/75 (76%) patients discontinued or reduced analgesic drug dosages on day 1 after the procedure. Postprocedural pain was controlled with weak opioid analgesics (9 patients) or nonsteroidal anti-inflammatory drugs (18 patients); no analgesic therapy was necessary for the remaining 30 patients. The mean analgesic scale decreased from 3.47 (SD, 1.31) before the procedure to 1.83 (SD, 2.04) postprocedure (P < .001). Furthermore, in both groups, the mean VAS, ODI, and KPS scores at each follow-up were significantly different from baseline scores (P < .05; Online Supplemental Data). Meanwhile, the changes in KPS, VAS, and ODI scores were not significantly different between patients with lesions in the 3 different zones and those treated via the 4 different approaches (P > .05).
DISCUSSION
The spine is the most common site of skeletal metastases, with 1%–7% of all skeletal metastases being in the sacrum.20 Sacral metastases reduce mechanical support and result in intractable radiating pain, often associated with immobility and a severely impaired quality of life. Currently, there is no consensus on the best treatment for sacral metastases. Traditional treatments, which include chemotherapy, surgery, targeted drugs, and radiation therapy, can kill tumor cells and reduce pain severity, but they act slowly and do not compensate for bone loss.21⇓⇓-24 Moreover, >80% of patients with sacral metastases have an advanced tumor stage and poor physical condition, making them ineligible for surgical treatment.25
PSP is a minimally invasive procedure that has been proved to effectively repair bone defects, inactivate metastatic cells, and relieve pain in patients with osteoporotic insufficiency or pathologic fractures in the sacrum.1,5⇓-7 The present study—comprising 126 patients with 162 metastatic lesions—is one of the largest studies to date on the use of PSP to treat sacral metastases; the previously published largest study was on a cohort of 42 patients.2 Consistent with previous reports, we found that PSP with or without RFA could provide immediate and lasting pain relief and allow functional recovery in patients with lesions in any part of the sacrum; significant improvement was noted in the VAS, ODI, and KPS scores after treatment with both PSP alone and PSP plus RFA.
With advances in technology, PSP continues to evolve. Modified PSP can be applied with balloon dilation, ablation, screw fixation, and high-viscosity cement augmentation.26⇓⇓⇓-30 Balloon dilation immediately after needle placement creates an easily accessible cavity that will allow more cement filling.26 Radiofrequency sacroplasty brings a low rate of leakage with highly viscous cement insertion and good results with regard to pain reduction.27 Lee et al28 reported that PSP combined with cryoablation could effectively treat sacral tumoral pain refractory to medical therapy. Andresen et al29 compared balloon sacroplasty with radiofrequency sacroplasty and concluded that both can enable reliable cement augmentation and achieve equally good clinical outcomes in the medium term. Contrary to the results of the present study, a previous meta-analysis indicated that these technical modifications were not associated with increased or decreased VAS study effect size compared with standard PSP.30
In theory, RFA before PSP can decrease tumor cell spread and the risk of cement extravasation by destroying viable tumor cells and embolizing venous channels; this result can be especially important in patients with a long life expectancy. Therefore, the combination of ablation and PSP is often recommended. In the present study, we found significantly better improvement in overall pain and mobility in patients treated with PSP plus RFA than in patients treated with PSP alone, indicating that PSP and RFA may act synergistically to provide pain relief. RFA can efficiently destroy tumor cells, decrease needle tract implantation metastases, and help avoid bone cement extravasation.11 The analgesic effect of RFA is due to destruction of pain-carrying nerves and tumor cells that produce pain-intensifying cytokines and growth factors. Meanwhile, PSP can provide immediate bone consolidation and anesthesia. This synergistic action is especially important for patients with a relatively long life expectancy. We believe that PSP plus RFA should be the preferred treatment for painful sacral metastases, regardless of the medical expense.
Because of the complex anatomy of the sacrum, the technique of PSP continues to evolve. Currently, the safest guidance system for interventional treatment is angiography with integrated CT, which enables precise puncture and real-time monitoring of the cement injection. In this study, we chose fluoroscopic guidance mainly because it allows real-time imaging during both PSP and RFA; it is especially useful during the cement-filling process. Except for 1 case of cement leakage that required surgery, none of the remaining patients experienced major complications, confirming the safety and reliability of fluoroscopic guidance.
Currently, there are 4 different approaches for PSP: transiliac, interpedicular, anterior-oblique, and posterior (which include the long-axis technique and the short-axis technique).31⇓⇓-34 In the present study, the efficacy and safety of the different approaches were similar. Because it is the practice in our institution for examinations and interventional procedures to be scheduled during 1 hospitalization, a prolonged hospital stay was observed, and the mean hospital stay was 4.51 (SD, 1.69) days in this study.
The main limitations of the present study are its single-center nature, lack of comparison with conservative or surgical treatments, and the heterogeneity of the metastatic tumor site and size. In addition, providers who clinically assessed patients were not blinded to PSP with or without RFA. This feature may also be a confounding factor in drawing more objective conclusions.
CONCLUSIONS
PSP, used alone or in combination with RFA, is safe and effective for the treatment of sacral metastases not responding to conservative treatments. The combination of PSP and RFA may provide better pain relief and mobility improvement. Large multicenter prospective studies are required to confirm these results.
Footnotes
This work was sponsored by grants from the National Natural Scientific Fund of China (grant No. 81701798, 81703751), the Natural Fund from Shanghai Science and Technology Commission (grant No. 19411971800), and the Natural Fund from Shanghai Municipal Health Commission (grant No. 202040340).
All the funding played important roles in design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received April 1, 2022.
- Accepted after revision June 7, 2022.
- © 2022 by American Journal of Neuroradiology