Abstract
BACKGROUND AND PURPOSE: Percutaneous thermal ablation followed by vertebral augmentation is an emerging minimally invasive therapeutic alternative for the management of spinal metastases. This study aimed to retrospectively evaluate the effectiveness and safety of microwave ablation combined with vertebral augmentation for the treatment of painful vertebral metastases.
MATERIALS AND METHODS: Overall, 91 patients with 140 metastatic vertebrae who experienced refractory moderate-to-severe pain were treated with CT-guided microwave ablation and vertebral augmentation. Procedural effectiveness was determined using the visual analog scale, daily morphine consumption, and the Oswestry Disability Index preprocedurally and during follow-up. Local tumor control was assessed at follow-up imaging.
RESULTS: The procedure was technically successful in all patients. The median visual analog scale score and mean morphine dose were 6 (range, 4–10) and 77.8 (SD, 31.5) mg (range, 15–143 mg), preprocedurally; 5 (range 3–8) and 34.5 (SD, 23.8) mg (range, 0–88 mg) at 3 days; 4 (range, 2–7) and 28.7 (SD, 16.4) mg (range, 0–73 mg) at 1 week; 3 (range, 1–6) and 24.6 (SD, 13.2) mg (range, 0–70 mg) at 1 month; 3 (range, 1–6) and 21.70 (SD, 10.0) mg (range, 0–42 mg) at 3 months; and 3 (range, 1–8) and 21.0 (SD, 9.9) mg (range, 0–46 mg) at 6 months postprocedurally (all P < .05). A decrease in the Oswestry Disability Index score was also observed (P < .01). Local control was achieved in 94.8% of the treated metastatic vertebrae during the 6-month follow-up period. Asymptomatic cement leakage occurred in 42 (30%) treated vertebrae. A grade 3 neural injury was observed in 1 patient (1.1%). The patient’s neurologic function returned to normal following treatment with mannitol, glucocorticoids, and radiation therapy.
CONCLUSIONS: This study demonstrates that percutaneous CT-guided microwave ablation combined with vertebral augmentation is a safe and effective minimally invasive intervention for the treatment of painful spinal metastases.
ABBREVIATIONS:
- MWA
- microwave ablation
- ODI
- Oswestry Disability Index
- RT
- radiation therapy
- VA
- vertebral augmentation
- VAS
- visual analog scale
Vertebrae are the most common bone metastatic sites because of their highly vascularized anatomy. Spinal metastases occur in up to 30% of patients with terminal cancer1 and frequently cause severe pain. Osteolytic spinal lesions are more likely to cause pathologic fractures and spinal cord compression. Neurologic injury and disability can occur via direct tumor compression or pathologic fractures; this issue greatly affects the patient’s quality of life.2
Radiation therapy (RT) is the mainstay of treatment for vertebral metastases.3 However, it has several limitations. First, certain tumor histologies, such as sarcoma, renal cell carcinoma, non-small cell lung cancer, and melanoma, respond less to RT.4 Second, 50% of patients who initially respond to RT experience relapse within a year,5 and re-irradiation is limited by the cumulative tolerance of the spinal cord. Finally, there is an increased risk of pathologic fracture following stereotactic body radiation therapy, with a reported incidence of 11.9%.6 Traditionally, surgery is preferred in patients with spinal instability or spinal cord compression;7 however, surgical procedures are invasive and may not be suitable for patients with poor performance status.
Percutaneous thermal ablation followed by vertebral augmentation (VA) is an emerging, minimally invasive therapeutic alternative for the management of spinal metastases. Studies on the combination of thermal ablation and VA for the treatment of painful spinal metastases have demonstrated satisfactory outcomes in terms of pain management and local control.8⇓⇓⇓-12 This retrospective study aimed to evaluate the effectiveness and safety of combined microwave ablation (MWA) and VA for the palliative treatment of painful spinal metastases.
MATERIALS AND METHODS
This study was approved by Tengzhou Central People’s Hospital Affiliated with Jining Medical University, institutional ethics committee. Institutional review board approval was obtained for a retrospective analysis. Informed consent was waived for the study. Overall, 91 patients (50 men, 41 women; mean age, 62 [SD, 11] years; range, 36–78 years) with 140 metastatic vertebrae underwent percutaneous MWA and VA at our institution between December 2016 and April 2020. The baseline clinical characteristics of the patients are listed in Table 1. Twelve (13.2%) patients with persistent or recurrent pain after RT received the treatment. Treatment locations were distributed almost evenly between the thoracic (n = 71, 50.7%) and lumbar regions (n = 69, 49.3%).
Baseline characteristics of the patients
The inclusion criteria were as follows: 1) pathologic evidence of primary cancer or vertebral metastasis; 2) recurrent or persistent pain after RT or radioresistant tumor histologies; 3) pain (visual analog scale [VAS] score, >4) that severely affected the patient’s quality of life; 4) ≤4 lesions under treatment per patient; and 5) life expectancy >3 months and a high grade in the Eastern Cooperative Oncology Group performance status (<3).
The exclusion criteria were as follows: 1) uncorrected coagulopathy (platelets, <50 × 109/L or international normalized ratio, >1.50); 2) uncontrolled infection around the surgical site or active systemic infections; 3) tumors with margins approximating the nerve roots; and 4) symptomatic spinal cord compression.
Preprocedural Evaluation
All patients underwent CT and MR imaging of the whole spine within 1 week before the procedure. Images were analyzed to determine the vertebrae to be treated, the degree of vertebral body compression, axial extension of the lesion, and whether the tumor involved the posterior vertebral body. Risks and complications were evaluated as well.
Treatment Procedure
The patient was instructed to lie on the CT table in a prone or lateral position. The location raster was placed on the back, and CT of the spine (section thickness, 0.75 mm) was performed, followed by 3D reconstructions. Images were analyzed at a workstation to plan the puncture site and approach. The patients were under conscious sedation with intravenous infusion of sufentanil (50 ug/mL diluted 1:10 with saline solution); local anesthesia (lidocaine hydrochloride 1% and ropivacaine hydrochloride 0.25%) was administrated.
A 13-ga bone needle was inserted into the center of the lesion or the vertebra with vertebral body compression using a transpedicular or transcostovertebral approach under 3D reconstruction CT guidance. When there were large lesions encompassing two-thirds of the vertebral body, 2 needles were inserted into the lesion through bilateral approaches for overlapping ablation zones and better cement distribution (n = 6). An MWA antenna (1.6 mm × 20 cm; ECO Microwave Electronic Institute) was coaxially inserted into the lesion following which the bone needle was retracted to expose the antenna with the antenna tip 1.5 cm beyond the bone needle. In lesions situated close to neural structures (n = 53), a 16-ga thermocouple needle was inserted and placed in proximity to the neural structure to monitor real-time temperature during MWA. Thermoablation was discontinued in case the temperature exceeded 42°C.
The MWA power was set between 20 and 40 W (mean, 29.3 [SD, 4.39] W) and was applied for a duration of 2–5 minutes (mean, 3.48 [SD, 1.36] minutes). The parameters of each ablation were selected depending on the location and size of the lesion. Preclinical data provided by the manufacturer showed that the mean diameter of the MWA area is close to 3 cm when the output power is 40 W. Ablation was performed in the form of short (30–90 seconds), repeat microwave cycles, and the clinical target volume was treated for improved local control. Consensus recommendations defined clinical target volume as the gross tumor volume along with abnormal marrow signals suspicious for microscopic invasion on MR imaging and adjacent normal bony expansion to account for the subclinical tumor spread in the marrow space.13
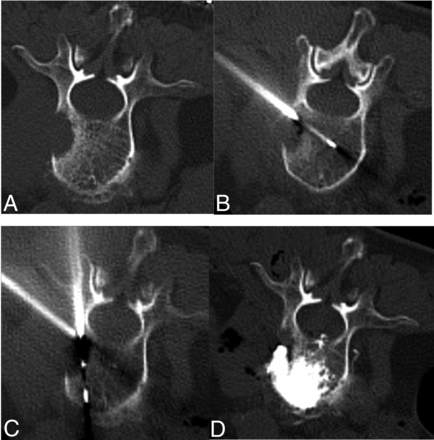
After ablation, the bone-puncture needle was advanced to the distal aspect of the tumor. Polymethylmethacrylate bone cement (Osteopal V; Heraeus) was prepared in a mixer. Several 1-mL syringes were used to extract the cement in its early paste phase; the extract was placed in iced physiologic saline to prolong the solidification time. VA was performed via the same access cannula. CT scans were repeated after each injection of 1 mL of cement to obtain a precise analysis of the cement distribution. A single vertebral body was scanned each time, and the scanning time was approximately 3 seconds. The cement was reduced to 0.2–0.5 mL whenever the cross-sectional CT images showed the cement approximating to the posterior edge of the vertebral body or neuroforamen (<0.5 cm). Injection was immediately terminated when CT images showed cement leakage into the spinal canal or intervertebral foramen. The mean volume of bone cement injected per lesion location was 5.4 (SD, 2.4) mL (range, 2–8) mL. A postoperative CT scan was obtained to examine the filling portion and bone cement leakage (Fig 1). All procedures were performed as inpatient procedures, and the average inpatient stay was 1–2 days.
A 68-year-old woman with painful osteolytic L2 metastases from lung adenocarcinoma was treated with MWA combined with VA. A, Preoperative axial CT shows L2 osteolytic destruction. B, A thermocouple needle was inserted and placed in proximity to the posterior vertebral body to monitor real-time temperature during MWA via the paravertebral approach. C, The MVA antenna is inserted into the lesion via a transpedicular approach. D, Postprocedural axial CT images show cement distribution in the treated vertebra.
Outcome Assessment
The VAS score was used to assess patients’ pain levels. The VAS involved a standard pain scale from 0 to 10 (0 = no pain, 10 = the most severe intolerable pain). Each patient’s daily opioid consumption was calculated as morphine equivalence. The quality of life was assessed using the Oswestry Disability Index (ODI). Pain score, daily morphine consumption, and ODI were obtained 1 day before the procedure and 3 days, 1 week, 1 month, 3 months, and 6 months postoperatively via follow-up visits or telephone interviews. After each procedure, patients were evaluated for any evidence of complications. Complications were graded using the Common Terminology Criteria for Adverse Events, Version 5.0.14
CT and MR imaging were performed at the 1-, 3-, and 6-month follow-up visits. Images were examined by an experienced radiologist and an interventionalist with >6 years of experience. Common consensus was achieved. Local tumor control was defined as no evidence of tumor progression. Local tumor progression was defined as follows: 1) increased osteolysis or paravertebral tumor extension; 2) new or persistent enhancing soft tissue extending into the epidural space, neural foramina, or paravertebral space; and 3) persistent fluorodeoxyglucose uptake on PET/CT.9
Statistical Analysis.
All statistical analyses were performed using the SPSS 19.0 statistical and computing software (IBM). Descriptive values of variables were expressed as mean (SD) or medians (minimum-maximum). The Kolmogorov-Smirnov test was used to determine the normal distribution of data. The Wilcoxon signed-rank test was used for group comparisons. Statistical significance was set at P < .05.
RESULTS
Technical success, defined as accurate placement of the antenna in the lesion, achievement of the target ablation power and time, and placement of adequate cement in the lesion, was achieved in 100% of the 140 metastatic vertebrae. One (1.1% ) patient who developed a neural complication received RT after the procedure. The other patients didn't receive RT after the procedure. All patients completed the 3-month follow-up. Eighty-eight patients completed the 6-month follow-up. Three patients died between 3 and 6 months after treatment. The patients died from heart attack, diffuse liver metastasis, and progression of an upper thoracic spinal metastasis that was not previously treated with the procedure, respectively. Fifty-three patients (59.6%) underwent MWA and VA for a single vertebra, 30 patients (33.7%) had 2 lesions, 5 patients (5.6%) had 3 lesions, and 3 patients (3.4%) had 4 lesions. The numbers of treated osteolytic metastases and mixed metastatic vertebrae were 114 (81.4%) and 26 (18.6%), respectively. Posterior vertebral wall defects were observed in 49 (35.0%) vertebrae due to tumor involvement or fractures. Vertebral pathologic compression fracture was present in 52 (36.6%) lesions.
On postprocedural CT images, the percentage of the lesion filled with bone cement was >50% in all vertebrae. Fifty-two (37.1%) metastatic vertebrae were completely filled with cement. Cement leakages were detected in 30% (42/140) of patients, localized in the intervertebral disk in 21.7% (12/140), the epidural space in 4.3% (6/140), the paravertebrae in 14.3% (20/140), the foramina in 2.1% (3/140), and the access track in 2.9% (4/140) of patients. Radiologic and operative characteristics are shown in Table 2.
Radiologic and operative characteristics
Effectiveness Assessments
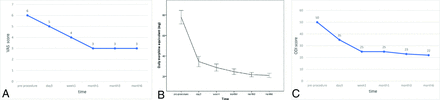
The median VAS scores were 6 (range, 4–10) preoperatively, 5 (range, 3–8) at 3 days, 4 (range, 2–7) at 1 week, 3 (range, 1–6) at 1 month, 3 (range, 1–6) at 3 months, and 3 (range, 1–8) at 6 months postoperatively. The differences between the median preprocedural and postoperative pain scores were statistically significant (P < .05) (Fig 2A). Pain reduction was obtained in 86% (78/91) of patients at 3 days, 88% (80/91) at 1 week, 92% (84/91) at 1 month, 92% (84/91) at 3 months, and 89% (78/88) 6 months postprocedurally. The mean daily morphine consumption equivalent of opioids was also reduced from 77.8 (SD, 31.5) mg (range, 15–143) preprocedurally to 34.5 (SD, 23.8) mg (range, 0–88) at 3 days, 28.7 (SD, 16.4) mg (range, 0–73) at 1 week, 24.6 (SD, 13.2) mg (range, 0–70) at 1 month, 21.70 (SD, 10.0) mg (range, 0–42) at 3 months, and 21.0 (SD, 9.9) mg (range, 0–46) at 6 months postprocedurally (P < .05 for all) (Fig 2B).
A, Changes in the median preoperative and postoperative VAS scores (P < .05 versus baseline). B, The mean (SD) of daily morphine consumption before and after the procedure. C, Changes in the median preoperative and postoperative ODI scores (P < .01 versus baseline).
The median ODI was 50 (range, 18–92) preprocedurally, 35 (range, 10–85) at 3 days, 25 (range, 9–55) at 1 week, 25 (range, 2–64) at 1 month, 23 (range, 3–56) at 3 months, and 22 (range, 4–80) at 6 months postprocedurally. The differences between the median preprocedural ODI score and postoperative scores at 3 days, 1 week, 1 month, 3 months, and 6 months were statistically significant (P < .01) (Fig 2C).
Follow-up with CT or MR imaging at 1 and 3 months after the procedure was available in all patients, and none of the patients experienced local tumor progression during this period. Imaging at 6 months after the procedure was available in all the surviving patients (88/91), and radiographic local control was achieved in 94.8% (128/135) of the treated metastatic vertebrae. No pathologic fractures at the treated vertebral levels were observed during the 6-month follow-up period.
Safety Assessments
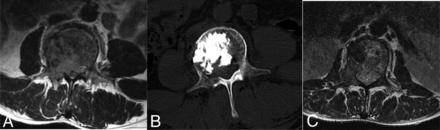
Complications occurring during the procedure were graded using the Common Terminology Criteria for Adverse Events, Version 5 .0. A grade 3 neural injury was observed in 1 patient (1/49, 2.0%) with epidural compression, who developed partial hemiplegia (3/5 motor strength) after the procedure. This patient underwent intermittent MWA with 30 W for 4.5 minutes, and a total of 7.2 mL of cement was injected into the metastatic vertebra. Postprocedural images showed residual tumor in the epidural space compressing the spinal cord and no leakage of cement into the spinal canal (Fig 3). After the procedure, the patient was treated with mannitol (125 mL, IV, 1 pill every 8 hours for 3 days), glucocorticoids (methylprednisolone, 200 mg/day IV for 3 days, then reduced by 20% every 3 days), and RT (30 Gy in 10 fractions). The patient’s neurologic function was normal 1 month after RT.
A 54-year-old man with painful L2 metastases from choroidal melanoma was treated with MWA and VA and developed partial hemiplegia after the procedure. A, An aggressive destructive osteolytic lesion of the L2 vertebra with posterior vertebral wall involvement and epidural compression is seen on the axial MR image. B, Sagittal CT shows the distribution of cement in the metastatic vertebra. C, Six-month follow-up axial MR imaging shows the epidural tumor shrinkage, and local tumor control was achieved.
Grade I cement leakages were present in 42 (30.0%) treated vertebrae. Skin burns, infection, bone cement embolism, hematoma, and periprocedural death were not observed. No pathologic fractures were observed during the 6-month follow-up period.
DISCUSSION
MWA has emerged as a newer ablation technique and an addition to the arsenal of minimally invasive cancer care. Thermal ablation causes coagulation necrosis of tissue within the ablation zone, which decreases the production of nerve-stimulating cytokines and destructs pain nerve fibers in the periosteum and bone cortex.15 However, MWA cannot increase the structural stability of the affected vertebral body. VA alleviates mechanical pain by treating compression fractures, microfractures, and instability.16 A combination of MWA and VA is advantageous because the cavitation after ablation promotes cement distribution in the lesion, and the combined treatment is more effective in terms of pain relief and structural stabilization.17,18
Clinical evidence for MWA and VA in the treatment of spinal metastases was limited to several small studies. Khan et al8 reported that follow-up imaging in patients surviving at 20–24 weeks demonstrated no locoregional progression; pain reduction was observed at 2–4 weeks and 20–24 weeks postprocedurally. Pusceddu et al17 reported MWA and cementoplasty of 35 osseous metastases, which included spinal lesions in 9 patients. Local tumor control was achieved in all patients at the 3-month follow-up. The mean reductions in the VAS score were 84%, 90%, and 90% at 1 week, 1 month, and 6 months, respectively.15 Wu et al18 reported 23 adult patients (33 high thoracic vertebral metastases) treated with MWA and VA. The mean VAS score, morphine consumption doses, and ODI decreased at 24 hours and 1, 4, 12, and 24 weeks postoperatively. Imaging showed no local tumor progression during the 24-week follow-up. The studies suggest that MWA and VA were highly effective in terms of pain alleviation and local tumor control. Our results were in accordance with those of previously reported studies.
MWA is more effective than radiofrequency ablation of high-impedance tissue such as bone and seems to be less affected by the surrounding tissue,12 resulting in deeper penetration, faster heating of tumors, and a short ablation time.19 The mean MWA time was 3.48 (SD, 1.36) minutes per level in our study, whereas a prospective study showed that the radiofrequency ablation procedure required 9.56 (SD, 4.58) minutes.20 There are radiofrequency ablation probes that can be curved in multiple directions to provide optimal tumor access, particularly in the central posterior vertebral body where access may be challenging using straight electrodes.21 MWA antennae are straight; thus, it was occasionally difficult to achieve adequate ablation for lesions in the central posterior vertebral body.
For fluoroscopy-guided VA, real-time visualization facilitates cement injection in a short time and an immediate recognition of cement extravasation.22 The main disadvantage of fluoroscopy is that the lesion being treated is often not visible. Under CT guidance, precise CT images are obtained to improve the view of metastasis and the correct positioning of the needle. Therefore, dual guidance with CT and fluoroscopy remains the best option in the combined treatment of vertebral metastases.23 In this study, we performed the procedure under CT guidance alone, injected small amounts of cement each time, and repeated CT scanning to observe precise cement distribution and leakage. Even though 30% of the patients with cement leakage were asymptomatic, blind cement injection still presents a high risk of extravasation, which may result in nerve compression. Moreover, repeat scanning leads to a high radiation dose to the patient. Studies with CT fluoroscopy will be undertaken in the future to decrease cement leakage and radiation.
Percutaneous thermal spine tumor ablation poses an inherent risk of injury to the spinal cord and nerve roots because of the proximity of the ablation zone to susceptible neural elements.24 Overheating of surrounding neural structures could possibly lead to severe complications during ablation. Some measures were adopted to ensure safety. The most common thermoprotective technique was the application of temperature-monitoring devices.8 Other thermoprotective techniques include perineural and epidural injections of carbon dioxide or 5% dextrose in water.9 Some studies suggest the use of low power and repeat short ablation cycles (30–90 seconds) to control diffusion of the heat zone without diminishing the effectiveness of MWA.8,25
In our study, we adopted a technique of low ablation power and short ablation cycles to ensure safety. In 53 cases, the lesions were close to neural structures, and a thermocouple needle was placed in proximity to the neural structure to monitor the real-time temperature during MWA. No neural injury related to thermal ablation occurred. In 1 patient, residual tumor in the epidural space compressed the spinal cord further after cement injection, causing partial hemiplegia after the procedure. The patient’s neurologic function returned to normal after treatment with drugs and RT. Mannitol and glucocorticoids alleviated spinal cord swelling. RT caused retraction of the epidural tumor. Therefore, the injury was attributed to spinal cord compression.
An inherent limitation of this study was the retrospective analysis of patient data without a control group. A higher level of evidence could be achieved by conducting a prospective, multicenter trial. Moreover, potential bias may have existed in this study because patient pain and disability could have been affected by potential additional metastatic disease, progression of the primary tumor, or additional systemic therapy.
CONCLUSIONS
To the best of our knowledge, this is the largest study on MWA combined with VA for the treatment of spinal metastases. This study demonstrates the effectiveness and safety of MWA with VA for the treatment of metastatic vertebrae. This combined treatment is a feasible and promising alternative for the treatment of spinal metastases and merits further exploration.
Footnotes
Disclosures: Dongliang Hou—RELATED: Statistical analysis. Lili Chen—RELATED: Medical writing, Payment for Writing or Reviewing the Manuscript, Funding acquisition. Kaixian Zhang—RELATED: Provision of study materials, supervision. project administration, Payment for English Language Editing. Guoliang Hou—RELATED: Data collection, data curation. Zhen Li—RELATED: Data collection. Sen Yang—RELATED: Participation in the procedure. Yuanyuan Qiu—RELATED: Participation in the procedure. Qianqian Yuan—RELATED: Participation in the procedure. Xin Ye—RELATED: Supervision.
This work was supported by faculty research fund of Jining Medical University, JYFC2018FKJ142.
References
- Received March 28, 2021.
- Accepted after revision September 9, 2021.
- © 2022 by American Journal of Neuroradiology