Abstract
BACKGROUND AND PURPOSE: Acute cerebellitis is an acute neurologic condition attributable to a recent or concurrent infection or a recent vaccination or ingestion of medication, with MR imaging evidence of cerebellar edema. MR imaging can confirm an anatomic abnormality and may allow the radiologist to establish a differential diagnosis. The purpose of this research was to evaluate the MR imaging findings in children with acute cerebellitis due to infectious versus immune-related conditions, in particular whether MR imaging findings allow differentiation.
MATERIALS AND METHODS: Electronic medical records were reviewed between 2003 and 2020 in our quaternary children’s hospital. Data included demographics and clinical records: presentation/symptoms, final diagnosis including acute cerebellitis and immune-related acute cerebellitis, length of stay, treatment, condition at discharge, and laboratory findings. Retrospective independent review of all brain MR imaging studies was performed.
RESULTS: Forty-three patients (male/female ratio, 28:15) were included in this study. Average age at presentation was 7.08 years (range, 0.05–17.52 years). Thirty-five children had infectious and 8 children had immune-related acute cerebellitis. Significant differences in neuroimaging were the following: 1) T2-FLAIR hyperintense signal in the brainstem (37.50% versus 2.85%, P = .016); 2) T2-FLAIR hyperintense signal in the supratentorial brain higher in the immune-related group (37.50% versus 0.00%, P = .004); and 3) downward herniation, higher in the infectious acute cerebellitis group (42.85% versus 0.00%, P = .03).
CONCLUSIONS: Acute cerebellitis is a rare condition, and MR imaging is helpful in the differential diagnosis. T2-FLAIR hyperintense signal in the brainstem and supratentorial brain may be indicative of immune-related acute cerebellitis, and downward herniation may be indicative of infectious acute cerebellitis.
ABBREVIATIONS:
- AC
- acute cerebellitis
- ACA
- acute cerebellar ataxia
- ADEM
- acute disseminated encephalomyelitis
- CE
- contrast enhancement
- IQR
- interquartile range
- WBC
- white blood cells
Acute cerebellar ataxia (ACA) and acute cerebellitis (AC) are often used incorrectly and interchangeably in the literature.1⇓-3 Ataxia refers to an impaired ability to coordinate voluntary movements, impairing a person’s ability to walk, talk, eat, and use fine motor skills in varying degrees.2 ACA is a term used for a clinical constellation with a heterogeneous etiology. Not all children with ACA undergo imaging routinely; therefore, the exact percentage of AC in children with ACA is unknown.4 The largest published series reported that AC occurred in 10% of children with ACA.5
Per definition, AC is an acute neurologic condition attributable to a recent or concurrent infective illness due to a virus or bacteria, a recent vaccination, or ingestion of medication, with MR imaging evidence of predominantly cerebellar edema.1 The incidence of AC is uncertain because most of the existing literature is limited to single case reports or smaller case series.1 Symptoms of AC are variable from classic symptoms of ACA to simple irritability, headache, photophobia, nuchal rigidity, or vomiting.6,7 In addition, seizures, focal neurologic deficits, fever, and altered mental status are reported.7 The most severe cases of AC may present with increased intracranial pressure due to severe cerebellar edema and resultant obstructive supratentorial hydrocephalus with serious morbidity and mortality. Diagnosis can be challenging in these cases. CSF examination is not always available in AC due to the marked swelling and impending herniation, though pronounced pleocytosis and elevation of proteins may be present when testing is available.7
Neuroimaging plays an important role in the diagnostic work-up of children with AC. MR imaging can confirm anatomic abnormalities involving the cerebellum and allow the radiologist to establish a differential diagnosis.7 Previous MR imaging studies in single patients showed varying imaging features in children with AC.1 However, no definitive imaging feature was described, depending on the exact etiology. Differentiation is essential for selecting the correct treatment and predicting outcome. The purpose of this research was to evaluate the MR imaging findings in children with AC due to infectious (parainfectious, postinfectious) versus immune-related conditions, in particular whether the imaging findings allow differentiation between etiologies.
MATERIALS AND METHODS
Following institutional review board approval, a retrospective review of the MR imaging studies was performed among children (younger than 18 years of age) diagnosed with AC between 2003 and 2020 in our quaternary Texas Children’s Hospital. A query search for MR imaging studies using the keywords “cerebellitis” and “ataxia” was performed in the PACS. Electronic medical records were reviewed for demographics (age, sex), clinical records (presentation/symptoms, final diagnosis including infectious AC and immune-related AC [acute disseminated encephalomyelitis, ADEM], hemolytic uremic syndrome, anti-N-methyl D-aspartate receptor encephalitis), length of stay, treatment, condition at discharge, and laboratory findings including white blood cells (WBC) in the complete blood count and CSF and glucose and protein levels in the CSF. Only the MRIs with a confirmed infectious AC or immune-related AC diagnosis were included in the study.
MR imaging studies of the brain were performed using standard departmental protocols on a 1.5T or 3T MR imaging scanner that included precontrast axial and sagittal T1-weighted turbo spin-echo, axial and/or coronal FLAIR, axial and coronal T2-weighted, axial gradient echo, axial DWI, and postcontrast axial and coronal T1-weighted turbo spin-echo imaging. Section thickness varied between 3 and 4 mm depending on the sequence.
A retrospective independent review of all brain MR imaging studies was performed by a board-certified pediatric neuroradiologist (S.F.K., with 9 years of experience) and a radiologist with pediatric neuroradiology research experience (G.O., with 8 years of experience). For all patients in whom there was a discordant MR imaging finding, the reviewers completed a secondary review to reach consensus. The consensus reading was used for final diagnosis.
MR imaging at initial presentation/diagnosis and follow-up MR imaging studies were evaluated for the following: 1) distribution of involvement of the cerebellum (unilateral, bilateral), brainstem, and/or supratentorial brain regions; 2) involvement of gray and/or white matter; 3) signs of compression and edema, effacement of fourth ventricle, effacement of the posterior fossa cisterns/subarachnoid spaces, downward or upward herniation, and supratentorial hydrocephalus; 4) contrast enhancement (CE); and 5) DWI characteristics, including vasogenic-versus-cytotoxic edema.
All statistical analysis was calculated using SAS/STAT software (https://www.sas.com/en_us/software/stat.html). All variables were assessed for normality. Comparisons between infectious versus immune-related AC groups for age at presentation were evaluated by unpaired t tests; complete blood count and CSF findings and length of stay were evaluated by the Wilcoxon signed rank test. Comparisons between infectious versus immune-related AC groups for MR imaging findings at presentation were evaluated by a 2-tailed Fisher exact test. Comparisons of MR imaging findings between presentation and follow-up studies for infectious versus immune-related AC groups were evaluated by the McNemar test. A P value < .05 was considered statistically significant.
RESULTS
A total of 2211 MR imaging and electronic medical records fulfilled the initial electronic search criteria for the study. Forty-three patients (male/female radio, 28:15) could be included in this study. There were 35 children with infectious (para-infectious and post-infectious) AC. The verified infectious agents were 2 HSV, 2 mycoplasma, 1 West Nile virus, 1 varicella virus, 1 enterovirus, 1 adenovirus, 1 influenza A virus. In 15 children no infectious agents were verified by testing, but they were diagnosed clinically during the acute infection and 11 children were post-infectious cases. Eight patients had immune-related AC (6 cases of ADEM, 1 hemolytic uremic syndrome, and 1 anti-N-methyl D-aspartate receptor encephalitis). A final diagnosis of an infectious AC agent was made by viral culture from the skin lesions in 2 patients with herpes simplex virus who had negative blood and CSF serology findings; by positive immunoglobulin M for 2 cases of mycoplasma; by polymerase chain reaction of the CSF and positive immunoglobulin M for 1 case of West Nile virus; by positive immunoglobulin M for 1 case of varicella virus; by polymerase chain reaction of blood in 1 case of enterovirus; by polymerase chain reaction of blood in 1 case of adenovirus; and by nasal swab in 1 case of influenza A virus. In addition, CSF culture and blood culture all had negative findings for these cases.
The average age at presentation was 7.08 years (range, 0.05–17.52 years); no statistically significant difference was found between infectious (mean, 7.19 [SD, 4.64] years) and immune-related AC (mean, 6.63 [SD, 5.60] years) groups (P = .77). Presenting symptoms in the infectious AC group were the following: 1) ataxia (n = 17), 2) altered mental status (n = 7), 3) headache (n = 9), and 4) seizure (n = 2). In the immune-related AC group, symptoms were the following: 1) ataxia (n = 4), 2) altered mental status (n = 3), and 3) seizure (n = 1). The median/interquartile range (IQR) for length of stay at our hospital was 8.0 days (5–13 days) days. No statistically significant difference was found between the infectious AC, 8 days (5–13 days), and immune-related AC, 7.5 days (5.5–19.5 days), groups (P = .49). Treatment with steroids was significantly higher in the immune-related AC, 100% (n = 8), than in the infectious AC group, 51.5% (n = 17) (P = .01). Twenty-one children were discharged after complete recovery; 20 patients were discharged after partial recovery, 12 of whom required occupational and/or physical therapy after their discharge. No significant difference was found between the infectious 27.3% (n = 9) and immune-related AC 37.5% (n = 3) groups (P = .67). Two patients did not have any treatment or follow-up records available for review.
The median/IQR for CSF WBC (0–5/cu mm) was 22.5 (2–62)/cu mm; no statistically significant difference was found between the infectious (27 [2–62]/cu mm) and immune-related AC (8 [9–135]/cu m) groups (P = .55), despite both groups having elevated CSF WBC values. The median/IQR CSF glucose level (40–70 mg/dL) was 56.0 mg/dL (50.0–64.0 mg/dL). No statistically significant difference was found between the infectious (57 [50–64] mg/dL) and immune-related AC (52 [49–66] mg/dL) groups (P = .99). The median/IQR for CSF protein (15–45 mg/dL) was 29.5 mg/dL (18–59 mg/dL). No statistically significant difference was found between the infectious (27 [18–64] mg/dL]) and immune-related AC (35 [24–52] mg/dL) groups (P = .77). The median/IQR for blood WBC (5.0–14.5 103/UL) count was 10.72 103/UL (7.55–14.83103/UL). No statistically significant difference was found between the infectious (10.7 [7.8–14.2] 103/UL) and immune-related AC (12.2 [7.1–21.5] 103/UL) groups (P = .55). Urine tests for toxicology analysis were available for 15/43 (11/35 in infectious AC and 4/8 in immune-related AC) patients, which all had negative findings, 100% (15/15) in both groups.
All patients had brain MRIs (mean, 1.79; range, 0–8 days) following their initial presentation. The average age at first MR imaging was 7.09 years (range, 0.05–17.53 years). Only 19 (infectious AC = 15, immune-related AC = 4) patients had follow-up brain MRIs with a mean interval of 234.8 days (range, 3–1193 days) between the first and last MR imaging studies.
MR imaging findings at presentation for both the infectious and immune-related AC groups are summarized in the Table. Significant differences in imaging findings comparing both groups were the following: 1) The hyperintense signal percentage in the brainstem on T2-weighted and FLAIR sequences was higher in the immune-related AC group than in the infectious AC group (37.50% [n = 3] versus 2.85% [n = 1], P = .016); 2) the hyperintense signal percentage in the supratentorial brain (1 patient had subtle ill-defined T2 and FLAIR hyperintensity in the white matter adjacent to the trigone of the left lateral ventricle in the left parietal region; another patient had ill-defined T2 and FLAIR hyperintensity seen in the white matter of the right frontal lobe posteriorly, the left parietal region superiorly, and the left frontal lobe; and finally the last patient had extensive multifocal and confluent bilateral, slightly asymmetric, T2 and FLAIR hyperintensities involving the cerebral white matter of both cerebral hemispheres including subcortical white matter, deep white matter and periventricular white matter and the gray matter, including the bilateral basal ganglia and bilateral thalami, slightly greater on the right side than left) in T2-weighted and FLAIR sequences was higher in the immune-related AC group than in the infectious AC group 37.50% (n = 3) versus 0.00% (n = 0) (P = .004); and finally 3) the downward cerebellar herniation percentage was higher in the infectious AC group than in the immune-related AC group (42.85%, n = 15, versus 0.00% n = 0, P = .03) (Table). No statistically significant difference was found for CE or DWI characteristics when comparing the infectious and immune-related AC groups (Table).
Brain MR imaging findings at presentation between infectious (n = 35) and immune-related (n = 8) acute cerebellitis subgroups
Comparisons of MR imaging findings between presentation and follow-up were calculated. Only downward herniation was significantly decreased between presentation (47.37%, n = 9) and follow-up (10.53%, n = 2, P = .016), though a clear trend for a decrease in cerebellar white matter involvement (36.84%, n = 7, versus 10.53%, n = 2), supratentorial hydrocephalus (31.58%, n = 6, versus 5.26%, n = 1), and leptomeningeal CE (43.75%, n = 7 versus 12.50%, n = 2) was also observed (P = .063). Cerebellar atrophy on follow-up MRIs was found in 15.79% (n = 3) of patients.
Representative cases are demonstrated in Figs 1–6.
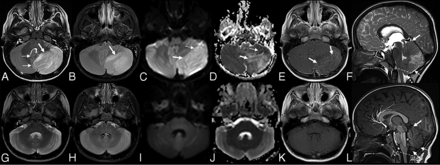
Unilateral cerebellitis in a 6-year-old boy who presented with ataxia with increased WBC on blood tests (infectious acute cerebellitis). Lumbar puncture was not performed. MR imaging acquired at presentation (upper row, A–F) shows unilateral left cerebellar gray matter and diffuse T2- and FLAIR hyperintensity (A and B, thick arrow), causing a shift of midline structures and displacement of the vermis (A, thin arrows). DWI (C) and an ADC map (D) show vasogenic (C and D, thick arrows) and cytotoxic edema (C and D, thin arrows). Leptomeningeal contrast enhancement is appreciated on postcontrast T1-weighted imaging (E, thick arrows). Sagittal T2-weighted imaging (F) shows the swollen cerebellum with upward (F, thick arrow) and downward (F, dashed arrow) herniation, supratentorial hydrocephalus, and effacement of the fourth ventricle (F, asterisk) and posterior subarachnoid spaces (F, thin arrow). Last follow-up MR imaging, which was acquired after 68 days, shows near-complete resolution of brain MR imaging findings (lower row, G–L) and atrophy. Note that sagittal T1-weighted imaging (L) shows the complete resolution of swollen cerebellum without any upward (L, thick arrow) and downward herniation (L, dashed arrow), no supratentorial hydrocephalus, and no effacement of the fourth ventricle (L, asterisks) and posterior subarachnoid spaces (L, thin arrow).
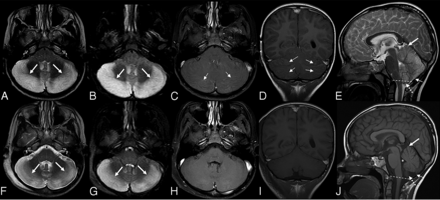
Bilateral cerebellitis in a 7-year-old boy who presented with ataxia and headache. He had a history of appendectomy 2 weeks before presentation (infectious acute cerebellitis). Blood tests at the time of imaging revealed WBC within normal limits. There were low total protein levels in the CSF, which was tapped 1 day before. The CSF culture had negative findings. A head CT (not shown) demonstrated diffuse swelling of the bilateral cerebellar hemispheres with crowding at the level of the foramen magnum with supratentorial hydrocephalus. Brain MR imaging acquired a day later (upper row, A–E) shows diffuse bilateral hyperintense signal on T2 (A, thick arrows) and FLAIR (B, thick arrows) sequences with corresponding leptomeningeal enhancement (thin arrows) on postcontrast axial (C) and coronal (D) T1-weighted imaging. Sagittal T2-weighted imaging (E) shows the swollen cerebellum with upward (E, thick arrow) and downward (E, dashed arrow) herniation, supratentorial hydrocephalus, and effacement of the fourth ventricle (E, asterisk) and posterior subarachnoid spaces (E, thin arrow). Follow-up MR imaging (lower row, F–J) acquired after 8 days shows improvement of hyperintense signal on axial T2 (F, thick arrows) and FLAIR (G, thick arrows) sequences. No contrast enhancement is seen on postcontrast axial (H) and coronal (I) T1-weighted imaging. Sagittal T1-weighted imaging (J) shows complete resolution of cerebellar edema, no upward (J, thick arrow) and downward (J, dashed arrow) herniation, no supratentorial hydrocephalus, and no effacement of the fourth ventricle (J, asterisk) and posterior subarachnoid spaces (J, thin arrow).
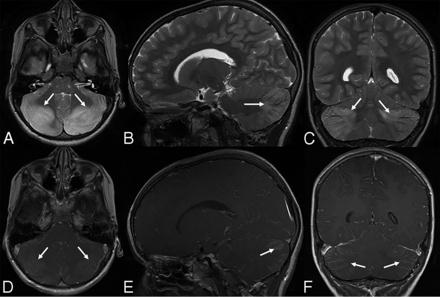
Acute disseminated encephalomyelitis in a 5-year-old girl who presented with ataxia and absent lower extremity reflexes (immune-mediated acute cerebellitis). Blood tests and CSF revealed an increased white blood cell count. CSF culture had negative findings. Brain MR imaging at presentation shows bilateral, diffuse, patchy hyperintense signal on axial T2 (A, arrows) and FLAIR (B, arrows) sequences. Corresponding vasogenic edema is seen on axial DWI (C, arrows) and ADC (D, arrows). Axial postcontrast T1-weighted imaging demonstrates bilateral, subtle leptomeningeal contrast enhancement (E, arrows). Consecutive supratentorial slices reveal subtle T2-hyperintense signal in the white matter of the adjacent trigone of the left lateral ventricle (F and G, arrow) without contrast enhancement or diffusion alteration (not shown). Sagittal T1-weighted imaging (H) shows no upward or downward herniation. Follow-up MR imaging was not available.
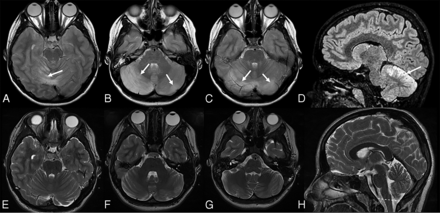
Bilateral cerebellitis in a 6.5-year-old boy (infectious acute cerebellitis) who had a history of gastroenteritis for 2–3 days. He developed marked and prolonged headache after a few days. Neurologic examination showed no cerebellar dysfunction. CSF examination was not performed. MR imaging shows bilateral, diffuse, T2 hyperintense signal and mild edema (A–C, arrows) in the cerebellar white and gray matter. Postcontrast imaging shows mild leptomeningeal contrast enhancement (D–F, arrows).
Bilateral cerebellitis in a 12.5-year-old girl (infectious acute cerebellitis). Initial presentation was nonspecific. The patient was tired with headache for a few days and spontaneously recovered. MR imaging at presentation (upper row) shows bilateral, diffuse, T2-hyperintense signal predominantly on the right side (A–D, arrows) and downward herniation (D, dashed line). A follow-up examination after 3 months shows no abnormal neurologic findings. Follow-up MR imaging after 3 months (lower row) shows complete resolution of previous MR imaging findings (E–H).
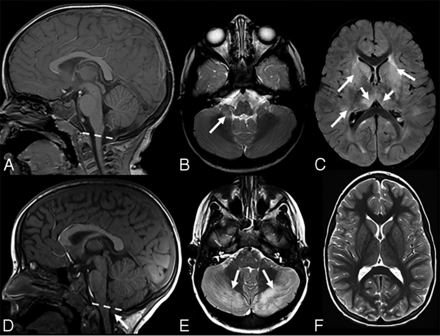
Upper row A–C, Acute disseminated encephalomyelitis in a 1-year 10-month-old boy (with immune-mediated acute cerebellitis) who presented with acute ataxia and fever with vomiting. A blood test revealed increased white blood cell count, and CSF revealed elevated WBC and normal protein and glucose levels. Blood culture and CSF culture had negative findings. Lower row D–F, Bilateral cerebellitis in a 5-year-old boy (with infectious acute cerebellitis) with a history dominated by intense headache, predominantly at night with some fluctuation. The patient had treatment of symptoms, but there was no clear improvement in headaches. MR imaging was performed about 2 weeks later. He had no cerebellar signs on examination. This figure aims to show the most predominant MR imaging findings for immune-mediated versus infectious acute cerebellitis. Note that there is no downward herniation in immune-mediated acute cerebellitis (A) versus cerebellar tonsil downward herniation in infectious acute cerebellitis (D, arrow). Axial T2 shows subtle hyperintense signal in right middle cerebellar peduncle in immune-mediated acute cerebellitis (B, arrow) versus bilateral diffuse T2-hyperintense signal with diffuse edema in infectious acute cerebellitis (E, arrows). Note that axial FLAIR shows extensive, multifocal, and confluent bilateral hyperintensity involving the cerebral white matter and the gray matter, including the bilateral basal ganglia extensively and bilateral thalami (C, arrows) in immune-mediated acute cerebellitis versus normal supratentorial brain axial T2 signal intensities in infectious acute cerebellitis (F).
DISCUSSION
AC is one of the main causes of ACA in childhood. The etiologies of AC can be subclassified as primary infectious (para-/postinfectious) versus immune-related (reactive/autoimmune).4,8 Certain MR imaging findings may be helpful to differentiate between infectious and immune-related AC. Our results show that T2-FLAIR hyperintense signal in the brainstem and/or supratentorial brain may be indicative of immune-related AC, and downward herniation may be indicative of infectious AC (Table).
Downward herniation was seen in almost half, and hydrocephalus, in almost one-quarter of our children with infectious AC. Yildirim et al9 reported a higher incidence of cerebellar herniation; however, with a similar incidence of hydrocephalus in their AC cohorts, De Bruecker et al10 reported hydrocephalus, and Kornreich et al11 reported tonsillar herniation and hydrocephalus. Bilateral cerebellar T2-FLAIR hyperintense signal was the most common finding in our infectious AC cases (Table). Yildirim et al, De Bruecker et al, and Kornreich et al reported similar MR imaging findings in their cases of AC. Unilateral involvement was less commonly observed in our patients. Involvement of 1 cerebellar hemisphere, also known as hemicerebellitis, is a rare variant of AC, and the mechanism for this preferential inflammation of 1 cerebellar hemisphere is unknown, though several hypotheses have been proposed, including impaired circulation or “subradiologic” involvement of the contralateral hemisphere.1,12 On MR imaging, the differential diagnosis of hemicerebellitis should include a posterior fossa tumor and acute ischemia.
Leptomeningeal and cortical CE was seen in our infectious AC cohort. Yildirim et al9 reported cortical and leptomeningeal CE, De Bruecker et al10 reported pial CE, and Kornreich et al11 reported CE in their patient groups. We did not find any statistically significant difference in CE patterns (leptomeningeal or cortical) between the infectious and immune-related AC groups (Table). However, leptomeningeal CE was a more frequent finding than cortical enhancement for both infectious and immune-related AC groups (Table). Pial enhancement has been reported previously.10 The authors concluded that if a lumbar puncture was performed before the contrast-enhanced MR imaging, definite differentiation from leptomeningeal enhancement due to the lumbar puncture or to the AC itself was not possible. MR imaging was performed after lumbar puncture in 15 patients with infectious AC and in 4 patients with immune-related AC in our study. This finding will likely explain the higher percentage of leptomeningeal CE in both AC groups.
We did not find any statistically significant difference for DWI characteristics (vasogenic or cytotoxic edema) between infectious and immune-related AC groups (Table). Vasogenic edema was common in both infectious and immune-related AC groups. Schneider et al13 found punctate areas of restricted diffusion in the cerebellum or cerebellar peduncles in patients presenting with acute and subacute onset of ataxia. We did not find any similar study to compare our DWI characteristics.
The differential diagnosis of infectious AC includes ADEM, Lhermitte-Duclos disease, diffusely infiltrating glioma or lymphoma, vasculitis, and drug-related causes.10 We compared MRIs of our infectious AC group with MRIs of children diagnosed with immune-related conditions and found the following: 1) significantly higher T2-FLAIR hyperintense signal in the brainstem in the immune-related AC group; 2) significantly higher T2-FLAIR hyperintense signal in the supratentorial brain in the immune-related AC group; and 3) significantly higher degrees of downward cerebellar herniation in the infectious AC group. Takanashi et al14 reported reversible splenial lesions (supratentorial hyperintense signal) in children with AC due to rotavirus infection. We did not find any other studies comparing infectious AC and immune-related AC groups. We suggest that our results may be helpful in the differential diagnosis of AC and may guide future studies.
Only 19 patients had follow-up brain MRIs, and cerebellar atrophy on follow-up brain MRIs was found in 15.79% of patients in our study. Hennes et al15 reported cerebellar atrophy on follow-up MR imaging in 36.36% of their patient group. This difference could be explained by a limited number of available follow-up MR imaging studies in our patient cohort.
Strengths of this study include the large number of patients and relatively homogeneous ages of patients (children). Limitations of this study are the following: 1) Due to the retrospective nature of the study, infectious agents could not be verified in all patients; 2) a discrepancy in size between the 2 patient groups might have affected statistical analysis and results; 3) a limited number of follow-up MR imaging studies might have affected statistical analysis and results; 4) this was a single-center evaluation of patients; 5) being a quaternary center may cause referral/selection bias of patients; and 6) study cases for inclusion were initially identified on the basis of a search of radiology reports.
CONCLUSIONS
AC is a rare condition, and MR imaging is helpful in the differential diagnosis. T2-FLAIR hyperintense signal in the brainstem and supratentorial brain may be indicative of immune-related AC, and downward herniation may be indicative of infectious AC. A decreased degree of downward herniation is the most significant MR imaging finding when comparing imaging at initial presentation and follow-up studies.
References
- Received March 22, 2021.
- Accepted after revision July 29, 2021.
- © 2021 by American Journal of Neuroradiology