Abstract
BACKGROUND AND PURPOSE: The ganglionic eminences are transient fetal brain structures that produce a range of neuron types. Ganglionic eminence anomalies have been recognized on fetal MR imaging and anecdotally found in association with a number of neurodevelopmental anomalies. The aim of this exploratory study was to describe and analyze the associations between ganglionic eminence anomalies and coexisting neurodevelopmental anomalies.
MATERIALS AND METHODS: This retrospective study includes cases of ganglionic eminence anomalies diagnosed on fetal MR imaging during a 20-year period from 7 centers in Italy and England. Inclusion criteria were cavitation or increased volume of ganglionic eminences on fetal MR imaging. The studies were analyzed for associated cerebral developmental anomalies: abnormal head size and ventriculomegaly, reduced opercularization or gyration, and abnormal transient layering of the developing brain mantle. The results were analyzed using χ2 and Fisher exact tests.
RESULTS: Sixty fetuses met the inclusion criteria (21 females, 24 males, 15 sex unknown). Thirty-four had ganglionic eminence cavitations (29 bilateral and 5 unilateral), and 26 had increased volume of the ganglionic eminences (19 bilateral, 7 unilateral). Bilateral ganglionic eminence cavitations were associated with microcephaly (P = .01), reduced opercularization, (P < .001), reduced gyration (P < .001), and cerebellar anomalies (P = .01). Unilateral ganglionic eminence cavitations were not significantly associated with any particular feature. Bilateral increased volume of the ganglionic eminences showed an association with macrocephaly (P = .03). Unilateral increased volume was associated with macrocephaly (P = .002), abnormal transient layering (P = .001), unilateral polymicrogyria (P = .001), and hemimegalencephaly (P < .001).
CONCLUSIONS: Ganglionic eminence anomalies are associated with specific neurodevelopmental anomalies with ganglionic eminence cavitations and increased ganglionic eminence volume apparently having different associated abnormalities.
ABBREVIATIONS:
- GA
- gestational age
- GE
- ganglionic eminence
The ganglionic eminences (GEs) develop in the ventral telencephalon, adjacent to the lateral ventricles during embryonic and early fetal life1 and are important proliferative zones that produce a wide variety of projection neurons and interneurons. Most important, they produce the cortical GABAergic interneurons that migrate tangentially to the neocortex.2,3 Recent studies have shown the complexity of the proliferative and migratory pathways from the GE, and some of the possible consequences of derangement in those processes include epilepsy, autism, and schizophrenia.4⇓⇓⇓-8 The human GE is visible on fetal MR imaging both ex vivo9 and in vivo, and recent case series by Righini et al10,11 have shown that GE anomalies can be shown during the late second and third trimesters.
Most GE anomalies fall into 2 broad categories: cavitations in the GE and increased volume of the GE; a range of associated structural brain anomalies have been described. The purpose of this exploratory study was to investigate the association between GE anomalies and coexisting cerebral developmental abnormalities in a large series.
MATERIALS AND METHODS
Case Selection
GE anomalies detected by fetal MR imaging are rare, so it was necessary to pool cases from a number of sources: 1) thirteen cases previously reported by Righini et al;10,11 2) twenty-one cases reported in a publication describing cortical formation anomalies from our collaborating network;12 and 3) twenty-six unpublished cases from our network identified after 2018. Appropriate ethics approval was obtained separately from the 6 centers in Italy and 1 in England. Specifically, if the fetal MR imaging study was performed for research purposes, informed consent from the woman was obtained before the MR imaging study, alternatively, if the fetal MR imaging was performed as a clinical examination, written consent was obtained from the woman retrospectively for use of the imaging data for the retrospective analysis. The MR imaging examinations were performed for either clinical or research purposes after expert sonographic evaluation with written informed consent from each woman in cases performed for research purposes. Cases with necrosis and/or hemorrhage in the GE (ie, increased T1-weighted signal/decreased T2*-weighted signal at echo-planar b = 0 images) or with evidence of associated acquired brain injury were not included because the aim of the study was to investigate associated developmental brain abnormalities, not generalized brain injury such as infection or hemorrhage.
MR Imaging Acquisition and Assessment
MR imaging protocols were not standardized across the centers due to the retrospective nature of the study and different MR imaging scanners at the sites, however, all studies were performed at 1.5T using abdominal or cardiac phased array coils. Each study included single-shot fast spin-echo T2-weighted sequences in 3 orthogonal planes (TE, between 80 and 180 ms; section thickness, between 3 and 4 mm; in-plane resolution, between 1.1 and 1.3 mm2) and axial T1-weighted sequences (FSE or gradient recalled-echo, 4- to 5-mm section thickness). All cases were consensus-reviewed by 3 senior pediatric neuroradiologists (A.R., C.P., P.D.G.), each with >15 years’ experience in fetal MR imaging. The presence of GE unilateral or bilateral cavitations or enlargement was recorded, along with details of any associated developmental brain anomalies (Online Supplemental Data). A cavitation in the GE region was defined as a small, well-demarcated ovoid or crescentic structure with CSF signal, lying between the GE and the adjacent parenchyma. Enlargement of the GE region was defined subjectively after a qualitative analysis on 3-plane images, taking into account a pool of cases with normal findings as a reference. The 2 main centers (1 in Italy and 1 in England) provided >70% of the collected cases and had access to a pool of fetal MR studies with normal findings ranging from 17 to 37 weeks’ gestational age (GA) (110 in Italy and 200 in England), which were used as reference cases. From those cases, we believe that the GE is visible between 17 and 30 weeks’ GA, though its prominence reduces with increasing maturity and is barely visible after the 30th week.
Head size was categorized on the basis of GA-matched reference centiles:13 microcephaly less than the third centile, normal size between the third and 97th centiles, and macrocephaly >97th centile. Other anomalies such as ventriculomegaly (mild, 10–12 mm; moderate-severe, >12 mm), agenesis or hypogenesis of corpus callosum, reduced opercularization, reduced gyration, cerebellar anomalies or hypoplasia, brain stem anomalies, abnormal transient layering of the developing brain mantle, unilateral or bilateral polymicrogyria, and hemimegalencephaly were recorded and included in the analysis.
Statistical Analysis
All variables are reported as median (interquartile range) unless stated otherwise. χ2 and Fisher exact tests were performed to assess the differences between the expected and observed frequencies of the associated cerebral developmental anomalies in fetuses with different types of GE anomalies. The Fisher exact test was selected over the χ2 test when the expected count in any cell of a 2 × 2 table was <5. A P value ≤ .05 was statistically significant. Statistical analysis was performed with SPSS statistical and computing software, Version 20 (IBM).
RESULTS
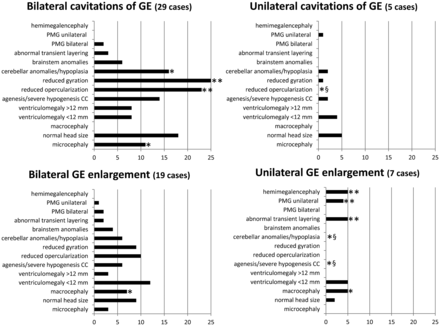
Sixty fetuses met the inclusion criteria (21 females, 24 males, and 15 sex not known; Online Supplemental Data). The average GA at MR imaging was 23.08 [SD, 3.5] weeks (range, 17–33 weeks). Thirty-four of 60 (57%) fetuses had GE cavitations (29 bilateral, 5 unilateral), and 26/60 (43%) had increased GE volume (19 bilateral, 7 unilateral). Figure 1 shows the type and frequencies of the associated brain anomalies.
Plots of the frequency of the associated brain anomalies for each of the 4 categories of GE anomaly reported. Asterisk indicates P <.05; double asterisks, P ≤ .001; section sign, a significant negative association. PMG indicates polymicrogyria; CC, corpus callosum.
Bilateral cavitations of the GE were associated with microcephaly (P = .01), cerebellar anomalies (P = .01), reduced opercularization (P = .001), and reduced gyration (P < .001). Unilateral cavitations of the GE did not show any positive, specific association with head size, while the negative correlation of the absence of reduced opercularization was significant (P = .01). Bilateral increased volume of the GE showed an association with macrocephaly (P = .03). Unilateral increased volume of the GE was associated with macrocephaly (P = .002), abnormal transient layering (P = .001), unilateral polymicrogyria (P = .001), hemimegalencephaly (P < .001), and significant absence of the following: agenesis or severe hypogenesis of the corpus callosum (P = .03), and cerebellar anomalies (P = .02).
DISCUSSION
GE abnormalities are a very rare finding on fetal MR imaging studies as highlighted by our ability to locate only 60 cases from 7 centers performing high numbers of fetal MR imaging studies during a 20-year period. Despite the rarity of a GE, the collaboration among the recruiting centers allowed sufficient numbers of cases of GE anomalies to uncover statistically significant associations between types of GE abnormalities and coexisting developmental brain abnormalities. Our categorization of GE abnormalities used a straightforward anatomic approach, describing either cavitations or increased size of the GE. We acknowledge, however, that there are problems in making subjective assessments of the size of structures such as the GE, whose borders may be somewhat indistinct. We also recognize the possibility that the GE can have reduced size, which could be associated with brain abnormalities. This article has not covered that subject, but it may be relevant for conditions such as primary microcephaly/microencephaly.
Within those limitations, we found that GE cavitations occur slightly more frequently than the increased size of the GE (34/60 versus 26/60), and each category of GE anomalies appears to be preferentially associated with specific types of brain abnormalities. When GE cavitations are present, they are much more likely to be bilateral rather than unilateral (29/36 versus 5/36). Bilateral GE cavitations were the most prevalent abnormality in our cohort and were most frequently associated with abnormalities of brain development, in the spectrum of microlissencephalies and cerebellar anomalies (Fig 2). The association between GE cavitations and cerebellar anomalies, albeit significant, is not readily explainable, but it is well-known that posterior fossa structures may be aberrant in microlissencephaly.
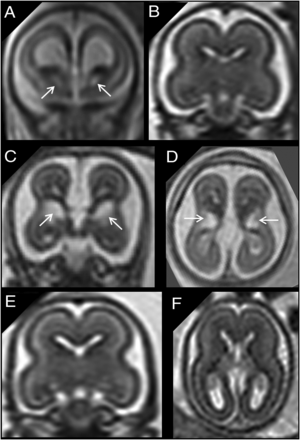
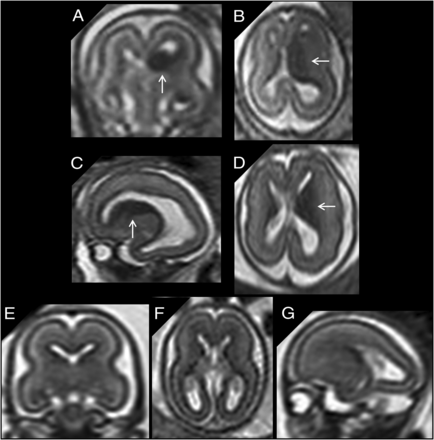
A, Coronal single-shot [SS]-FSE T2-weighted section of a 20 weeks’ GA fetus shows bilateral cavitation of the GE (arrows). B, A GA-matched control. C and D, Coronal and axial SS-FSE T2-weighted sections of a 21 weeks’ GA fetus showing bilateral cavitation of the GE regions (arrows). The fetus had microcephaly (less than the third centile), reduced opercularization and gyration (ie, parieto-occipital sulcus), brain stem and cerebellar anomalies (not shown), and agenesis of corpus callosum. E and F, A GA-matched control.
As reported previously,10,11 the bilateral, symmetric inverted C-shaped morphology of GE cavitations with a clear margin from the adjacent basal ganglia and the absence of high signal on T1-weighted images are highly associated with brain malformation. Familial recurrence of these cases has been reported10,11,14 and further supports the argument. Reduction in head size and delayed opercularization and gyration were also found to be associated with bilateral GE cavitations in our cohort. This association may be explained by disruption of normal cell proliferation and migration in and from the GE, mediated by a genetic mutation in one of the many genes involved in GE neurogenesis.7 At present, however, no pathophysiologic explanation has been demonstrated in human subjects, but it is likely that numerous genetic mutations will be discovered in the future. Five of 60 fetuses showed unilateral cavitation of the GE (Fig 3 and Online Supplemental Data): In this subgroup, there were no demonstrable positive associations with other developmental brain anomalies. Given the small number, this finding should be interpreted cautiously.
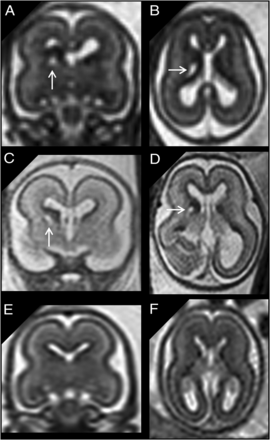
A and B, Coronal and axial single-shot [SS]-FSE T2-weighted sections of a 21 weeks’ GA fetus show unilateral cavitation of the GE (arrows). Brain size was normal, and no associated anomalies were found. C and D, Coronal and axial SS-FSE T2-weighted sections of another 21 weeks’ GA fetus show unilateral cavitation of the GE (arrows). Head size was normal, but an ipsilateral temporal-occipital large polymicrogyric area was found. E and F, A GA-matched control.
Nineteen of 60 fetuses had bilateral increased volume of GEs (Fig 4 and Online Supplemental Data), and we showed an association only with macrocephaly. This finding leads, however, to a hypothesis about an underlying mechanism involving excessive and abnormal neuroblast proliferation and migration. The overall lack of other associations suggests that the finding is not specific for a pathophysiologic process but can be found in a wide array of neurodevelopmental abnormalities, encompassing macrocephaly, opercularization and gyration anomalies, and cerebellar anomalies. It seems intuitive that in cases of a generalized brain size increase (ie, brain gigantism associated with a mammalian target of rapamycin kinase [MTOR] gene mutation), the GE volume may be concordantly enlarged as well (Fig 4 and Online Supplemental Data). The opposite condition is more difficult to explain, ie, when enlarged GEs are found in fetuses with small heads (present in 3 of our cases with bilateral and 1 with unilateral GE enlargement). Previous reports have linked the enlargement of the GE to reduced opercularization15 and Tubulin gene mutation–related lissencephaly (tubulin alpha 1a [TUBA1A] gene defect).16,17 As previously suggested,10 one of the hypothetic causes of an enlarged GE could be an excessive accumulation of neuroblasts in the GE due to a delay/arrest of the migration to the cortical destination or delay in the physiologic involution of the GE itself during neurogenesis. The animal model of the aristaless related homeobox (ARX) gene mutation,18 which is characterized by microlissencephaly and an enlarged GE during the fetal period, could support, at least in part, this hypothesis. A recently reported human fetal case with the ARX gene mutation and showing clear GE enlargement supports this view.19 Given the different morphologic features of the developing brain with bilateral increased volume of the GE, multiple pathophysiologic mechanisms are likely at play and specific genetic mutations are required to classify specific associated malformations, as also reported by Amir et al.20
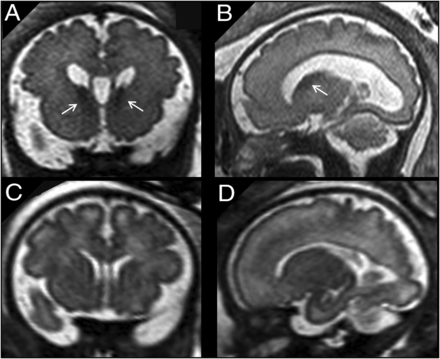
A and B, Coronal and sagittal single-shot-FSE T2-weighted sections of a 33 weeks’ GA fetus show bilateral increased volume/abnormal persistence of the GE (arrows). Macrocephaly (>97th centile) and mild ventriculomegaly (<12 mm) are also noted. An activating mutation of MTOR gene was identified. C and D, A GA-matched control.
Seven of 60 patients were found to have a unilateral increased volume of the GE (Fig 5), and 5 of those fetuses had hemimegalencephaly. We found a statistically significant association between unilateral enlargement of the GE and macrocephaly, abnormal transient layering, and unilateral polymicrogyria, but these are explained by the large number of cases with hemimegalencephaly in this subgroup. The explanation for the association between GE enlargements concordant with ipsilateral cerebral hemisphere enlargement is intuitive. Unilateral GE enlargement was also associated with the significant negative correlation of the absence of agenesis or hypogenesis of corpus callosum, reduced opercularization, and reduced gyration. All of these associations can be explained by the high prevalence of hemimegalencephaly and the characteristic MR imaging findings of this entity.21 The 2 cases without hemimegalencephaly had normal brain size and showed no other neurodevelopmental abnormalities. It is likely that these cases belong to a different pathophysiologic entity with a less severe phenotype and probably a rarer occurrence or, conversely, are more difficult to detect at prenatal screening.
A and B, Coronal and axial single-shot [SS]-FSE T2-weighted sections of a 21 weeks’ GA fetus show unilateral increased volume of the GE (arrows) with homolateral hemimegalencephaly. C and D, Sagittal and axial SS-FSE T2-weighted sections of another 21 weeks’ GA fetus showing unilateral increased volume of the GE (arrows). Associated anomalies were early-stage unilateral polymicrogyria and hemimegalencephaly. In both cases, the normal brain mantle layering for age was deranged in the enlarged hemisphere. E–G, A GA-matched control.
We elected not to correct for multiple tests (using, for example, the Bonferroni correction) because our study was not specifically hypothesis-directed, rather, it was an exploratory study with the view that future studies would use our current data to perform prospective, formally powered studies. Correction for multiple tests was not performed, to maximize the chance of finding possible associations in this exploratory study and hence minimizing the risk of false-negatives when correcting P values for multiple comparisons. We accept, however, that this approach will lead to an increased risk of type I errors.
Only 19/60 cases did not have any form of ventriculomegaly; this finding should be taken into account because prenatal sonographic detection of ventriculomegaly is one of the leading findings prompting MR imaging investigation. Thus, in our cohort, the real proportion of cases with GE anomalies but without ventriculomegaly might have been underestimated.
Our work suggests that GE abnormalities are central to a range of neurodevelopmental abnormalities that encompass abnormal neuroblast proliferation-differentiation and migration processes, which often lead to generalized or local abnormal neuronal organization. We recognize, however, that we have reported several associations that are not readily explainable with known etiologic and pathophysiologic data. For example, unilateral cavitations of the GE region were not specifically associated with a prevalent anomaly, hinting at a “milder” phenotype whose pathophysiology and prognosis could be very different from those in the fetuses with bilateral GE cavitations. Of note, all 5 fetuses with unilateral cavitation had normal head size.
Clinical follow-up studies and the results of genetic analysis are required in this field of work and will make an invaluable contribution to the definition of possible subgroups, which will allow improved counseling of pregnant women whose fetuses have GE abnormalities.
CONCLUSIONS
This study expands on the understanding of GE anomalies in the fetus and provides statistically validated insights into the associated neurodevelopmental anomalies. We cannot currently provide a specific etiologic/genetic classification of the GE and associated anomalies, but we believe that our data strengthen the current understanding and build a valid framework for future studies.
Footnotes
Disclosures: Mariasavina Severino—UNRELATED: Employment: neuroradiology consultant, Istituto Di Ricovero e Cura a Carattere Scientifico–Istituto Giannina Gaslini.
References
- Received October 26, 2020.
- Accepted after revision December 17, 2020.
- © 2021 by American Journal of Neuroradiology