Abstract
BACKGROUND AND PURPOSE: Few investigators have studied the lateral ventricle formation related to the development of the calcarine sulcus. Our purpose was to establish the relationship between the lateral ventricles and the calcarine sulcus in the second and third trimesters.
MATERIALS AND METHODS: Fetal brain MR imaging (3T and 7T) was performed in 84 fetuses at 14–35 gestational weeks. The lateral ventricles and calcarine sulcus were 3D-reconstructed, and quantitative measurements were obtained.
RESULTS: The lateral ventricle volume decreases slowly at 14–23 gestational weeks and then increases rapidly at 24–35 gestational weeks. The depth and length of the calcarine sulcus develop with the increase in gestational weeks, leading to be squeezed in the lateral ventricle posterior horn. A linear correlation occurs between the calcarine sulcus length and posterior horn length: Right-length = 2.4204 (LPH) − 27.5706, Left-length = 2.0939 (LPH) − 23.4099.
CONCLUSIONS: The variation of lateral ventricle volume evolved from a slow to rapid increase at 14–35 gestational weeks. The shrinkage in the lateral ventricle posterior horn is accompanied by the development of the calcarine sulcus, resulting in a better linear correlation between the calcarine sulcus length and the posterior horn length. The present results are valuable in elucidating the evolution of lateral ventricle development and provide clues for the diagnosis of lateral ventricle abnormalities in the prenatal examination.
ABBREVIATIONS:
- LAPP
- width between the anterior and posterior parts
- LBIH
- width between the bilateral inferior horns
- LCI
- length between the central part and inferior horn
- LHCP
- intraventricular height at the central part
- LPH
- length of the posterior horn
- LTAP
- total anteroposterior length
Fetal brain development is a highly complex and delicate process, during which the size and the shape of the brain change rapidly. The lateral ventricles occupy most of the fetal brain, and their changes are closely related to the changes in brain structures. At present, studies on the development of the lateral ventricles are focused on the second trimester, and the data on the third trimester are still lacking. It would be valuable to study the changes in the lateral ventricles within a wide range of gestational ages.
Many ultrasonographic and in utero MR imaging studies on the size of the lateral ventricles are limited to subjective descriptions and 2D measurements, which cannot reflect the size and the shape of lateral ventricles correctly.1 A number of recent studies have started using postmortem specimens to study the development in the lateral ventricles and have found that the changes in the lateral ventricles are related to the development of the surrounding structures during fetal development.2 The volumes of the basal ganglia and the ganglionic eminences increase with gestational weeks, whereas the volume of the lateral ventricles decrease later in the second trimester.3 Fetal total lateral ventricle and thalamus volumes were correlated at 22–38 gestational weeks.4 A recent study found that the inward folding and development of the neonatal cerebral surface were considered the main cause of the decrease in the volume of the lateral ventricles.5 It was observed that the changes in the lateral ventricles are consistent with not only the growth of internal surrounding structures but also the external cortical folding. The calcarine sulcus is an important and consistent sulcus in the occipital lobe, which is considered the most valuable landmark for the recognition of the medial surface of the occipital lobe.6 On the basis of the location correlation of the calcarine sulcus and the lateral ventricles, some scholars have pointed out that there is a certain relationship between them during their development.
Animal-based research confirmed that the decrease in ventricular volume was accompanied by an increase in the depth of the calcarine sulcus in cynomolgus monkey fetuses.7 The study concluded that the degree of infolding of the calcarine sulcus can be used as the anatomic landmark for evaluating the cerebral maturation. Then, the correlation investigation was conducted between the morphologic maturation of the calcarine sulcus and the width of lateral ventricles in human fetuses with isolated mild ventriculomegaly at 20–36 gestational weeks.8 The study indicated that there was a negative correlation between the fetal calcarine sulcus depth and the width of the lateral ventricles in fetuses with isolated mild ventriculomegaly. It was also found that the lateral ventricle volume began to decrease after the appearance of the calcarine sulcus in the gyral development of the human brain.9 Studies have depicted the relationship between the lateral ventricles and calcarine sulcus in animals and fetuses with isolated mild ventriculomegaly.7,8 This relationship is also fascinating and interesting in healthy fetuses and needs to be studied to further elucidate the evolution of the fetal brain. Thus, the present study planned to first investigate the development of the lateral ventricles and then explore the developmental correlation during the normal human fetal development across 14–35 gestational weeks using high-field-strength MR imaging.
Materials and Methods
Subjects
A total of 161 fetuses at 14–35 gestational weeks were selected from medically indicated or spontaneous abortions within 24 hours, fetal deaths attributed to maternal diseases, stillbirths caused by abnormal delivery, and premature deaths caused by diseases outside the brain such as respiratory diseases, from hospitals in the Shandong Province of China. According to the strict inclusion criteria that were established on the basis of the size of the cerebrum and the developmental status of the sulci, lateral ventricles, and corpus callosum in previous studies,10,11 84 specimens were included in this study to minimize the influence of brain deformation on experimental results. Previous studies have confirmed the clinical application of the volume and the sulcal width in fetuses with formalin fixation.12,13 Therefore, all the specimens were immersed in 10% formalin and scanned by MR imaging within 2 months after the fetal death.
The gestational age of fetuses at 14–35 gestational weeks was estimated according to their crown–rump length and/or pregnancy records and was expressed as weeks from the last menstrual period.14 The Table presents the number of subjects in each gestational week, including the numbers of males and females. The specific protocol of this study was approved by the Human Research Ethics Committees of the School of Medicine of Shandong University. The parents' consent to donate the fetal cadaver was obtained.
Distribution of gestational age and number of specimens in each age selected (n = 84)
Image Acquisition
The specimens were scanned by keeping the brain in situ without destroying the ventricular system and subarachnoid space to ensure that the extrauterine environment was consistent with the intrauterine environment of the lateral ventricles. We performed 3T and 7T MR imaging at 2 stages for clear images and accurate 3D reconstruction. We used 7T MR imaging to scan the fetus at 14–22 gestational weeks because the structure of the fetus was immature during this period. The 3T MR images of the fetal brain at 23–35 gestational weeks were sufficient to show the structural boundaries. Postmortem MR imaging ensures the consistency of measurement and the accuracy of results, avoiding the effects of motion artifacts, field strength limitations, and so forth.15 Finally, 41 specimens at 14–22 gestational weeks were scanned by 7T micro-MR imaging (70/16 PharmaScan; Bruker Biospin, Ettlingen, Germany). The inner diameter of the rat body coil used was 60 mm. The acquisition parameters of T1-weighted images were the following: TR/TE, 384.4/15.8 ms; matrix size, 512 × 512; slice thickness, 0.8 mm; number of excitations, 1; FOV, 6 × 6 cm; voxel size, 0.8 × 0.12 × 0.12 mm3. The acquisition parameters of T2-weighted slice images were the following: TR/TE, 17,000/50 ms; matrix size, 256 × 256; slice thickness, 0.5 mm; number of excitations, 4; FOV, 6 × 6 cm; voxel size, 0.5 × 0.23 × 0.23 mm3.14
Due to the coil limitations, 43 specimens at 23–35 gestational weeks were scanned with a Signa 3T MR imaging scanner (GE Healthcare, Milwaukee, Wisconsin). The acquisition parameters of T1-weighted slice images were the following: TR/TE, 2580.0/23.4 ms; matrix size, 512 × 512; slice thickness, 2 mm; number of excitations, 1; voxel size, 2 × 1.9 × 1.9 mm3. The acquisition parameters of T2-weighted slice images were the following: TR/TE, 4600.0/111.6 ms; matrix size, 512 × 512; slice thickness, 2 mm; number of excitations, 1; voxel size, 2 × 1.9 × 1.9 mm3.
Image Processing
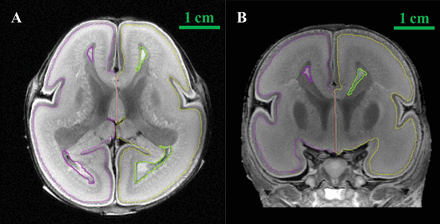
This work was performed using manual segmentation. The selected structures were segmented on horizontal, sagittal, and coronal planes using the inner interface in Amira 5.2.2 software (www.amira.com) (Fig 1). Segmenting these structures was performed manually by 2 experienced anatomists based on a histology atlas of second-trimester fetal brains.16 Then, the 3D reconstruction models were obtained as shown in Fig 2.
Segmentation of the brain structures with Amira 5.2.2. A, segmentation on the axial plane. B, Segmentation on the coronal plane.
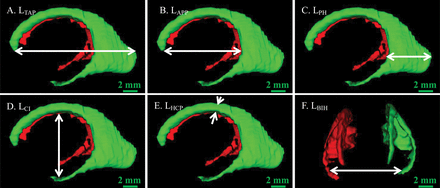
Length measurements of the lateral ventricle. Arrows indicate the length. A, LTAP. B, LAPP. C, LPH. D, LCI. E, LHCP. F, LBIH.
Data Measurement
The lateral ventricle volume was obtained by Amira software, and we measured the following lengths of the 3D ventricle model: total anteroposterior length (LTAP), width between the anterior and posterior parts (LAPP), length of the posterior horn (LPH), intraventricular height at the central part (LHCP), length between the central part and the inferior horn (LCI), and width between the bilateral inferior horns (LBIH) (Fig 2). These measures reflect the elongation and narrowing of the ventricles and also reflect the changes of surrounding structures around the lateral ventricles.2
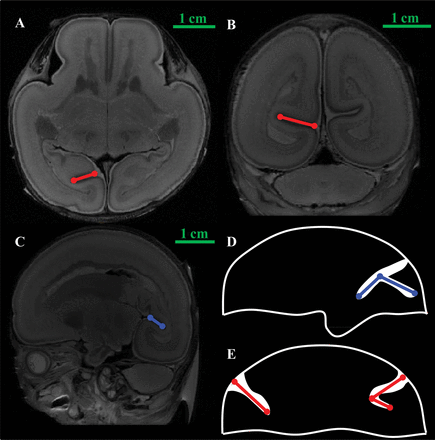
Sulcal depth is defined as the distance between the superficial and deep ridges (Fig 3A, -B). When the sulcus is curved, the sum of the paths is the sulcal depth (Fig 3E). The length of the calcarine sulcus was measured on the sagittal plane (Fig 3C). It is the sum of the anterior and the posterior parts of the calcarine sulcus (Fig 3D). For the sulcal depth and length, the maximum value on the multiple layers was obtained by 2 anatomists, and the mean value was used as the last measurement.
Measurement of the calcarine sulcus. A, The depth of the calcarine sulcus (axial plane). B, The depth of the calcarine sulcus (coronal plane). C, The length of the calcarine sulcus (sagittal plane). D and E, The measurements of the depth and the length in the calcarine sulcus. The blue line shows the length of the sulcus. The red line shows the depth of the sulcus.
Statistical Analysis
We used 3T and 7T MR imaging data to obtain high-precision segmentation and measurement data. Thus, all data were analyzed with SPSS software, Version 17.0 (IBM, Armonk, New York) in 1 group. Simple correlation analysis was used to analyze the correlation between the lateral ventricle posterior horn length and the measurement of the calcarine sulcus. A 2-sample t test for dependent samples was used to test sex differences. The differences in the hemispheres were compared using the paired-sample t test for dependent samples. P < .05 was considered statistically significant.
Results
Morphologic Evolution of the Lateral Ventricles
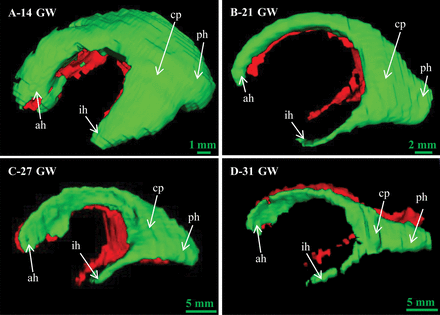
Figure 4 delineates the morphologic evolution of the lateral ventricles at 14–31 gestational weeks. The lateral ventricles were immature at 14 gestational weeks, and they gradually developed into 4 parts at 21 gestational weeks: the anterior horn, inferior horn, posterior horn, and central part. The anterior horn, inferior horn, and central part became thin at 27 gestational weeks. The posterior horn became flat at 31 gestational weeks.
The morphologic evolution of fetal lateral ventricles at 14–31 weeks. ah indicates anterior horn; ph, posterior horn; ih, inferior horn; cp, central part; GW, gestational weeks.
Lateral Ventricular Volume
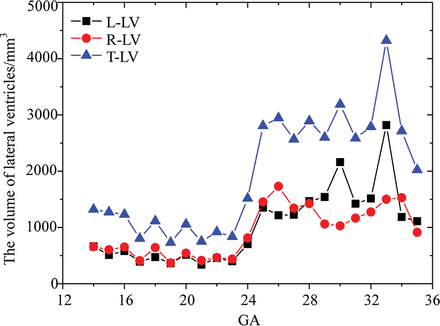
The left, right, and total ventricular volume were obtained after 3D reconstruction (Fig 5). The variation of ventricular volume can be divided into 2 stages: a stable stage at 14–23 gestational weeks and a fluctuant stage at 24–35 gestational weeks. The total volume of the lateral ventricles declined slowly at the stable stage and then increased to the first peak of 2945.68 mm3 at 26 gestational weeks. Then the lateral ventricle volume fluctuated from 26 to 35 gestational weeks, reaching the maximum value of 4321 mm3 at 33 gestational weeks and a low value of 2023.288 mm3 at 35 gestational weeks. After a slow descent, the left volume increased to the first peak of 1215.7 mm3 at 26 gestational weeks and then fluctuated twice until 33 gestational weeks, reaching the maximum value of 2820.015 mm3. The right volume was smaller than the volume of the left lateral ventricles at the fluctuant stage. The right volume decreased slowly at the stable stage and rose to 1729.98 mm3 at 26 gestational weeks. After a relatively stable plateau, the right volume reached 1527.73 mm3 at 33 gestational weeks and then dropped to 912.968 mm3 at 35 gestational weeks.
Volume measurements of the lateral ventricles. L-LV indicates the left lateral ventricle volume; R-LV, right lateral ventricle volume; T-LV, total lateral ventricle volume; GA, gestational age.
Length Parameters
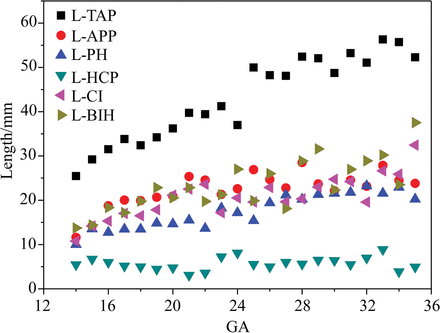
Figure 6 represents the variation of 6 length parameters with the gestational weeks, where the LTAP obviously increased from 25.46 to 52.28 mm, reflecting the development of the whole brain. There was no obvious change in the LHCP, which may correspond to the relatively stable development of the body of the corpus callosum.17,18 For the other 4 curves, the LBIH increased from 13.74 to 37.53 mm. A smaller magnitude of increase occurred in the LCI and LAPP, 10.785–32.45 and 11.61–23.81 mm, respectively.
Quantitative measurements of the lateral ventricles.
Depth and Length of the Calcarine Sulcus
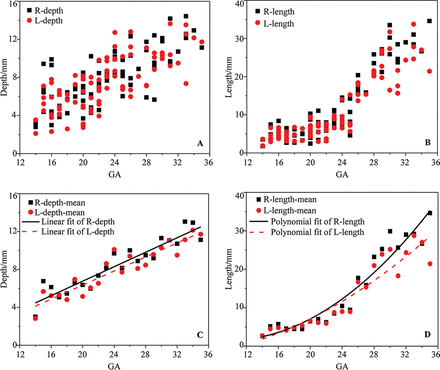
Figure 7 reflects the changes in the depth and length of the calcarine sulcus at 14–35 gestational weeks with an increasing trend. The distribution of the sulcus length was more concentrated than that of the depth, showing a smaller individual difference. The depth had a linear increase compared with the smooth variation in the length at 14–23 gestational weeks (Fig 7A). At 14 gestational weeks, the average depth reached 2.82 mm and then increased to 5.67 mm at 15 gestational weeks and 8.61 mm at 23 gestational weeks. At 24–35 gestational weeks, the average depth increased from 10.11 to 12.71 mm with a linear growth law (Fig 7C). The length development was nonlinear growth with the increase in gestational weeks, which was not consistent with the depth of the calcarine sulcus. After a platform period from 14–23 gestational weeks, the length of the calcarine sulcus increased rapidly (Fig 7D).
The depth and length of the calcarine sulcus. A, The depth of the calcarine sulcus. B, The length of the calcarine sulcus. C, The mean depth of the calcarine sulcus. D, The mean length of the calcarine sulcus.
Location Correlation
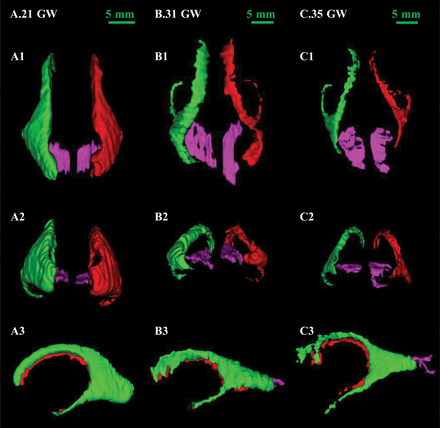
The calcarine sulcus and lateral ventricles were reconstructed in 1 space to show the spatial position relationship (Fig 8). The calcarine sulcus was close to the posterior part of the lateral ventricles (Fig 8A1, B1, and C1). The inner side of the calcarine sulcus was parallel to the cerebral median fissure. The lateral ventricles became thin with the increase of gestational weeks, and the calcarine sulcus became wider and longer. The posterior view showed that part of the 2 structures overlapped and the morphologic characteristics were not obvious. On the lateral view, the calcarine sulcus and lateral ventricles overlapped before 21 gestational weeks. With the development of the calcarine sulcus, the posterior part gradually extended longer backward. The occipital part of the lateral ventricles narrowed with the lengthening of the calcarine sulcus.
The location of the calcarine sulcus in 3D space: superior view (A1, B1, C1), posterior view (A2, B2, C2), lateral view (A3, B3, C3). A, Twenty-one GW (A1, A2, A3). B, Thirty-one GW (B1, B2, B3). C, Thirty-five GW (C1, C2, C3). The left lateral ventricle is green, the right lateral ventricle is red, and the calcarine sulcus is pink. GW indicates gestational weeks.
Measurements Correlation
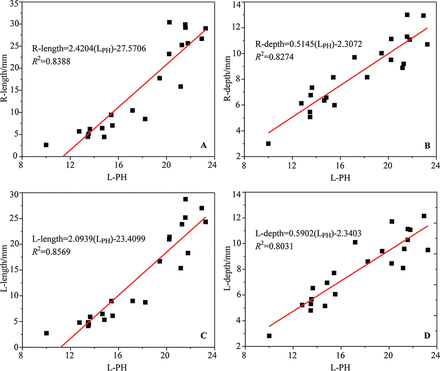
According to the results of location correlation, the length and depth of the left and right calcarine sulcus and the posterior horn length in the lateral ventricles were selected for analysis in this study. The depth and length of the calcarine sulcus were linearly related to the posterior horn length (Fig 9). The length and depth of the calcarine sulcus can be expressed by the following equation: Right-length = 2.4204 (LPH) – 27.5706, Right-depth = 0.5145 (LPH) – 2.3072, Left-length = 2.0939 (LPH) – 23.4099, Left-depth = 0.5902(LPH)–2.3403.
The correlation between the LPH and the depth and length of the calcarine sulcus.
Sex and Side Difference
There was almost no difference between the left and right lateral ventricle volume before 24 gestational weeks and a large difference after 24 gestational weeks (Fig 5). However, statistics showed that there was no hemispheric difference in the lateral ventricle volume during the entire period (P > .05). The depth and length of the calcarine sulcus in the right hemisphere were greater than those in the left, which was statistically significant (all P < .05). There were no sexual dimorphisms in those measurements (all, P > .05).
Discussion
Formation of the Fetal Lateral Ventricle
The morphologic evolution of the lateral ventricles is affected by many aspects such as the cortical folding and the development of subcortical structures.3 At 10–13 gestational weeks, the lateral ventricles account for most of the brain because of the vesicular period of the ventricular system.19 In this study, it was found that the lateral ventricle morphology still had the characteristics of the vesicular period at 14 gestational weeks (Fig 4A). The shape of the lateral ventricles evolves from an embryonic crescent into the immature lateral ventricles with 4 horns.20 With the development of the fetal brain, the lateral ventricles begin to lose the features of the vesicular period and gradually separates into 4 distinct parts at 16 gestational weeks.2 The increase in LAPP, LCI, and LBIH indicates the development of the basal nuclei and thalamus at this stage, resulting in the narrowing of the anterior horn of the lateral ventricles (Fig 4B, -C). It is evident that the slope of the LPH is less than that of the LTAP, findings in agreement with the shrinkage of the lateral ventricular occipital region (Fig 6, Fig 4D). Eventually, the lateral ventricle morphology development approaches that of adults.
The volume of the lateral ventricles decreased at 14–23 gestational weeks (Fig 5), which is in agreement with the study by Clouchoux et al.21 However, Huang et al3 found that the volume of the lateral ventricles first increased and then decreased at 13–21 gestational weeks. Kinoshita et al19 observed that the volume of the lateral ventricles increased at 7–23 gestational weeks. The results of the lateral ventricle volume are different in these studies. One reason is the differences in the sample sizes and individual fetuses. Another reason could be the differences in the structural anatomic definition and inclusion of the third ventricle or the cavum septum pellucidum. It is conceivable that the decrease in volume is associated with the change of the ventricular morphology. At 14 gestational weeks, the vesicular effect of the ventricles gradually disappears and the volume of the lateral ventricles is reduced correspondingly. Meanwhile, the volume of the germinal matrix and subcortical structures such as the basal nuclei also increases with the gestational week.22 It is inferred that the shrinkage in the lateral ventricles is accompanied by the expansion of the germinal matrix and basal nuclei. The lateral ventricles and these subcortical structures develop together and affect each other in the morphologic evolution of the lateral ventricles.
At 23–35 gestational weeks, the volume of the lateral ventricles increases with the fluctuating trend. The LTAP grows faster than the LAPP, LHCP, and LCI, reflecting the growth in the brain volume being faster than that of the basal ganglia, thalamus, and other structures around the ventricles (Fig 6). Thus, the lateral ventricle volume increases rapidly at 23–26 gestational weeks. This finding is in agreement with a previous study that found that fetal brain volume increases linearly and subcortical structures such as germinal matrix volume decrease at this stage.22 After 26 gestational weeks, the fetal brain volume increases quickly and the germinal matrix decreases. The germinal matrix gradually develops into the caudate, putamen, globus pallidus, and basal ganglia.3 The increase in LAPP, LBIH, and LCI represents these subcortical structures beginning to expand. Then the increased velocity of the lateral ventricle volume becomes slower. It is inferred that the individual differences in development are more obvious at this stage, resulting in the increase in the volume of the lateral ventricles with the fluctuating trend.
A difference occurred in the development of left and right lateral ventricle volume after 24 gestational weeks, which may be related to the lateralization of cerebral hemisphere development. Lateral differences in the cerebral hemispheres already exist at birth. The growth of the cerebral hemisphere structures may affect the lateral ventricles, resulting in this large difference in lateral ventricle volume. Meanwhile, this difference may also be related to other features such as the number of specimens and the method of measurement and so forth. However, the cerebral lateralization is a very complex problem that requires more data to reach a conclusion. The change in the lateral ventricle volume is stable at 14–23 gestational weeks and fluctuates after 23 gestational weeks. The lateral ventricle volume can reflect the development of the lateral ventricles comprehensively compared with the lateral ventricle width on a 2D level.4 It is suggested that the lateral ventricle volume measured by 3D sonography techniques needs to be used to improve prenatal diagnostic accuracy after 23 gestational weeks.
Correlation Analysis Between the Lateral Ventricles and the Calcarine Sulcus
The growth of the calcarine sulcus length accelerated noticeably after a slow developmental period. It is consistent with the variation of the lateral ventricle volume (Fig 7D). This finding indicates that there is a certain relationship between the calcarine sulcus and lateral ventricles. Thus, we elucidated the relationship between the lateral ventricles and the calcarine sulcus, including the location and measurement correlations.
The calcarine sulcus is located at the inner side of the lateral ventricle occipital part. The inner sides of the calcarine sulcus on both sides are parallel (Fig 8A1). The calcarine sulcus gradually increases backward and exceeds the occipital part of the lateral ventricles (Fig 8B1, -C1). The curvature of the posterior calcarine sulcus is related to the acceleration of cortical folding in the later developmental period (Fig 8A3–C3).5 The calcarine sulcus depth increases and extends outward. The lateral ventricle occipital part becomes flattened, and the size relatively decreases (Fig 8A3–C3).
Combined with the development of the fetal brain, the variation of calcarine sulcus length and the volume of lateral ventricles is associated with the whole-brain development.23 At 14–23 gestational weeks, the whole calcarine sulcus does not exceed the boundary of the lateral ventricle occipital part. The lateral ventricles and the calcarine sulcus gradually develop with fetal brain development. At 24–35 gestational weeks, the calcarine sulcus posterior part size exceeds the lateral ventricle occipital part and becomes a wave type. Both of them are in a relatively slow growth period; then, the growth becomes faster. The variation of the calcarine sulcus length and lateral ventricle volume is roughly consistent with the trend of fetal brain volume.
On the basis of the above analysis, the lateral ventricle occipital part is affected by the calcarine sulcus in its development. As the increase in the calcarine sulcus depth, the posterior horn length becomes smaller. There is a better linear correlation between the left calcarine sulcus length and the posterior horn length represented by the value of the LPH (Fig 9). The fitting effect between the LPH and calcarine sulcus length is better than that between the LPH and the calcarine sulcus depth, which is consistent with the phenomenon that the point dispersion degree of the calcarine sulcus depth is greater than that of calcarine sulcus length (Fig 9 and Fig 7A, -B). This feature indicates that the stability of the calcarine sulcus length is better than that of the calcarine sulcus depth. The linear correlation between the lateral ventricle and the calcarine sulcus is helpful to elucidate the development of the lateral ventricles and improve the prenatal diagnosis of fetal ventriculomegaly.
Conclusions
The lateral ventricle volume increases, with a slow-to-rapid growth period at 14–35 gestational weeks. The shrinkage in the lateral ventricle posterior horn is accompanied by the development of the calcarine sulcus, resulting in a better linear correlation between the calcarine sulcus length and the posterior horn length. The relevant results are helpful in understanding the evolution of the lateral ventricles and providing a basis for the diagnosis of lateral ventricle abnormality. Our future work is to study the relationship between the fetal lateral ventricles and the calcarine sulcus in patients with ventriculomegaly, to provide more valuable data for a differential diagnosis and prenatal examination of ventriculomegaly.
Acknowledgments
We thank Derong Duan, Qing Xia, Wenjia Liang, and Wenjun Wang for language help during the revision.
Footnotes
This work was supported by the National Natural Science Foundation of China (No. 31771328, 31571237).
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- Received October 27, 2018.
- Accepted after revision February 13, 2019.
- © 2019 by American Journal of Neuroradiology