Abstract
BACKGROUND AND PURPOSE: Low-field 64 mT portable brain MRI has recently shown diagnostic promise for MS. This study aimed to evaluate the utility of portable MRI (pMRI) in assessing dissemination in space (DIS) in patients presenting with optic neuritis and determine whether deploying pMRI in the MS clinic can shorten the time from symptom onset to MRI.
MATERIALS AND METHODS: Newly diagnosed patients with optic neuritis referred to a tertiary academic MS center from July 2022 to January 2024 underwent both point-of-care pMRI and subsequent 3T conventional MRI (cMRI). Images were evaluated for periventricular (PV), juxtacortical (JC), and infratentorial (IT) lesions. DIS was determined on brain MRI per 2017 McDonald criteria. Test characteristics were computed by using cMRI as the reference. Interrater and intermodality agreement between pMRI and cMRI were evaluated by using the Cohen κ. Time from symptom onset to pMRI and cMRI during the study period was compared with the preceding 1.5 years before pMRI implementation by using Kruskal-Wallis with post hoc Dunn tests.
RESULTS: Twenty patients (median age: 32.5 years [interquartile range {IQR}, 28−40]; 80% women) were included, of whom 9 (45%) and 5 (25%) had DIS on cMRI and pMRI, respectively. Median time interval between pMRI and cMRI was 7 days (IQR, 3.5−12.5). Interrater agreement was very good for PV (95%, κ = 0.89), and good for JC and IT lesions (90%, κ = 0.69 for both). Intermodality agreement was good for PV (90%, κ = 0.80) and JC (85%, κ = 0.63), and moderate for IT lesions (75%, κ = 0.42) and DIS (80%, κ = 0.58). pMRI had a sensitivity of 56% and specificity of 100% for DIS. The median time from symptom onset to pMRI was significantly shorter (8.5 days [IQR 7−12]) compared with the interval to cMRI before pMRI deployment (21 days [IQR 8−49], n = 50) and after pMRI deployment (15 days [IQR 12−29], n = 30) (both P < .01). Time from symptom onset to cMRI in those periods was not significantly different (P = .29).
CONCLUSIONS: In patients with optic neuritis, pMRI exhibited moderate concordance, moderate sensitivity, and high specificity for DIS compared with cMRI. Its integration into the MS clinic reduced the time from symptom onset to MRI. Further studies are warranted to evaluate the role of pMRI in expediting early MS diagnosis and as an imaging tool in resource-limited settings.
ABBREVIATIONS:
- cMRI
- conventional MRI
- DIS
- dissemination in space
- DIT
- dissemination in time
- IQR
- interquartile range
- IT
- infratentorial
- JC
- juxtacortical
- LMIC
- low- and middle-income countries
- pMRI
- portable MRI
- PPV
- positive predictive value
- PV
- periventricular
- pwMS
- patients with MS
- SN
- sensitivity
SUMMARY
PREVIOUS LITERATURE:
Access to MRI remains a barrier to early MS diagnosis, particularly in low- and middle-income countries. The use of portable brain MRI enables increased accessibility to MRI, and the potential clinical applications of pMRI has been increasing, especially in emergency and acute care settings. Recent studies have also demonstrated that white matter lesions can be detected by using low-field pMRI including in patients with MS.
KEY FINDINGS:
In 20 patients with new optic neuritis, pMRI enabled diagnosis of DIS in 5 of 9 patients (56%). Furthermore, it showed moderate concordance and high specificity for DIS compared with cMRI and reduced the time from symptom onset to MRI.
KNOWLEDGE ADVANCEMENT:
Integrating pMRI into clinical practice may complement cMRI by providing timelier neuroimaging at the point of care, which could facilitate early DIS diagnosis in patients presenting with typical demyelinating syndromes.
MS is the leading cause of nontraumatic disability in young and middle-aged adults. Early diagnosis with prompt initiation of disease-modifying therapy leads to reduced relapse rates and disability progression in patients with MS.1,2 Contemporary diagnostic criteria in MS are anchored on the principles of dissemination in space (DIS) and dissemination in time (DIT). MRI is currently the most useful paraclinical test to establish an MS diagnosis, substituting for clinical findings in the determination of DIS or DIT in patients presenting with typical symptoms. Specifically, MRI DIS criteria require the presence of at least 1 T2-hyperintense lesion in at least 2 of the following anatomic locations: periventricular (PV), cortical/juxtacortical (JC), and infratentorial (IT) brain regions, as well as the spinal cord. DIT criteria can be fulfilled by the simultaneous presence of enhancing and nonenhancing lesions at any time or by a new lesion on follow-up MRI with reference to a baseline scan.3 In a survey conducted by the MS International Federation between 2019 and 2020,4 lack of awareness of MS symptoms among the public and health care professionals and insufficient health care professionals with the necessary expertise to diagnose MS were reported as major barriers to early MS diagnosis. Furthermore, access to fixed conventional MRI (cMRI) remains an obstacle to early MS diagnosis in up to one-third of surveyed countries, particularly in low- and middle-income countries (LMICs).
Recently, low-field portable MRI (pMRI) has been shown to be a safe, cost-effective technology that enables point-of-care neuroimaging. It has been successfully deployed in intensive care units, emergency departments, as well as in resource-limited and geographically remote settings.5⇓⇓⇓–9 Its clinical applications include detection of intracranial hemorrhages,10,11 ischemic strokes,6,12,13 midline shift,14 hypoxic-ischemic brain injury,15 and assessment of optic chiasm decompression after endoscopic endonasal surgery.16 Furthermore, despite reduced SNR and lower spatial resolution compared with cMRI, pMRI has shown diagnostic promise for identification of white matter lesions in patients with MS (pwMS).17 In a recent study, pMRI was able to depict lesions in 31 of 33 (94%) pwMS with established lesions on 3T cMRI and had 100% sensitivity for lesions >5 mm.18 pMRI was also proved to have moderate agreement with cMRI for depicting moderate to severe leukoariosis.19
In this study, we evaluated the diagnostic utility of pMRI in determining DIS in patients presenting with optic neuritis, which is a common initial presenting symptom of pwMS and may be accompanied by disseminated white matter lesions in 50%–60%.20 We also evaluated whether deploying pMRI in the MS clinic can shorten the time interval from symptom onset to the MRI scan.
MATERIALS AND METHODS
Study Setting and Participants
This study was done as part of a quality improvement initiative at St Michael’s Hospital looking into image quality, accuracy, turnaround time, clinical impact, and cost associated with the utilization of 64 mT pMRI system (Swoop, Hyperfine) compared with conventional CT and MRI obtained in standard clinical care. This initiative was formally reviewed and approved by institutional authorities at Unity Health Toronto and deemed to neither require formal research ethics board approval nor written informed consent from participants. We performed a retrospective analysis of all consecutive patients referred to our academic MS center for newly diagnosed optic neuritis from July 2022 to January 2024 who underwent both point-of-care pMRI in the MS clinic and subsequent 3T cMRI (Magnetom Skyra, Siemens) in the radiology department. It is our institutional practice to scan all patients with suspected and confirmed MS on 3T machines to facilitate easier and accurate comparisons between scans.
Optic neuritis was considered based on visual acuity, Humphrey visual field testing, pupillary and optic nerve clinical information as evaluated by a fellowship-trained neuro-ophthalmologist (J.M.). All patients met criteria for optic neuritis published in the landmark Optic Neuritis Treatment Trial study.21 Alternative causes of retinal or optic nerve disease were ruled out, and objective changes of optic neuritis were confirmed with optical coherence tomography after 1 month.
MRI Protocols and Imaging Assessment
The pMRI examinations were performed as a clinical study at the time of the patient’s initial assessment in the MS clinic. The scans were conducted by a radiologic technologist in a clinical examination room either before or after the clinical assessment by an MS neurologist. Imaging was performed by using an 8-channel head coil by using the manufacturer’s standard protocol that included the following sequences: axial and sagittal T2-FLAIR (TR/TE/TI = 4000/234/1400 ms; in-plane resolution = 1.6 × 1.6 mm; slice thickness = 5 mm; acquisition time = 9:35 minutes per acquisition plane) and axial T2-FSE (TR/TE = 2000/243 ms; in-plane resolution = 1.5 × 1.5 mm; slice thickness = 5 mm; acquisition time = 6:50 minutes) with total scan time of approximately 26 minutes. All pMRI examinations were interpreted by a neuroradiologist at the time of scanning, and the results were readily available to the treating neurologist.
The cMRI examinations were acquired subsequently as an elective outpatient examination with a 20-channel head-neck coil by using our institutional demyelination protocol that includes a 3D T2-FLAIR sequence (TR/TE/TI = 4800/352/1800 ms; in-plane resolution = 1 × 1 mm; slice thickness = 1 mm; acquisition time = 6:26 minutes).
T2-FLAIR and T2-FSE pMRI images were reviewed by 2 fellowship-trained neuroradiologists (T.R.L. and S.S.) for the presence of PV, JC, and IT lesions. Raters were blinded to the cMRI findings and clinical report of pMRIs. Raters were not involved in the clinical reporting of the pMRI scans. Disagreements were adjudicated by a third observer experienced in pMRI interpretation (A.B.). Adjudicated ratings were also compared with the findings on the actual real-world clinical report of the pMRIs. DIS was determined on brain MRI according to the 2017 McDonald criteria.3 The size of the largest and smallest lesions on pMRI and cMRI were measured on T2-FLAIR sequences.
Time from Symptom Onset to First MRI
To assess whether the deployment of pMRI in the MS clinic can shorten wait times for MRI, the time from clinical onset of symptoms to either pMRI or cMRI was compared in patients presenting with new onset optic neuritis during our study period (n = 30) and in the preceding 1.5 years before pMRI implementation (January 2021 to June 2022; n = 50).
Statistical Analysis
Statistical analyses were done by using Stata Statistical Software: Release 14 (StataCorp). Test characteristics were computed by using cMRI as the reference standard. Interrater agreement as well as intermodality agreement between pMRI and cMRI were estimated by using Cohen κ (0.00–0.20 indicates poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; and 0.81–1.00, very good agreement). Mean lesion sizes were compared by using the Mann-Whitney U test. Time from symptom onset to pMRI or cMRI between the different groups was compared by using the Kruskal-Wallis test, with a post hoc Dunn test.
RESULTS
Patient and MRI Characteristics
A total of 20 patients underwent pMRI during the duration of study with median age of 32.5 (interquartile range [IQR], 28−40); 16 (80%) were women. Median time interval between pMRI and cMRI was 7 days (IQR, 3.5−12.5). PV lesions were seen in 8 (40%), JC in 4 (20%), and IT in 3 (15%) on 64 mT pMRI; McDonald criteria for DIS on pMRI was met in 5 (25%) of these patients. On fixed 3T cMRI, 10 (50%) had PV, 7 (35%) had JC, 8 (40%) had IT lesions, with 9 (45%) fulfilling DIS criteria. The mean size of the largest lesions identified on pMRI (10.4 ± 3.1 mm) and cMRI (8.4 ± 4.0 mm) was not significantly different (P = .24), but the mean size of the smallest lesions detected on cMRI (3.2 ± 0.9 mm) was significantly smaller than on pMRI (5.3 ± 1.0 mm, P < .01) (Table 1). Of the 9 patients who met DIS criteria on cMRI, 5 of 9 (55%) also satisfied DIT criteria for MS: 4 of 9 (44%) have gadolinium-enhancing lesions on cMRI and 1 of 9 (11%) was positive for CSF-specific oligoclonal bands. Orbital MRI was performed concurrent with cMRI of the brain in 18 of 20 (90%) patients, all of whom had findings consistent with optic neuritis. All patients also had objective findings of prior optic neuritis on optical coherence tomography done 1 month after.
Patient demographics and MRI characteristics
Interobserver and Intermodality Agreement
Interrater agreement was very good for PV lesions (95%, κ = 0.89), and good for JC and IT lesions (90%, κ = 0.69 for both); agreement for DIS was good (90%, κ = 0.69) (Online Supplemental Data). Similarly, the agreement between the raters in this study and the real-world clinical radiology reports of the pMRI scans was very good for PV (95%, κ = 0.89) and JC lesions (100%, κ = 1), good for IT lesions (90%, κ = 0.69), and very good for DIS diagnosis (95%, κ = 0.88) (Table 2).
Interrater and intermodality agreement
Between pMRI and cMRI, agreement was good for PV (90%, κ = 0.80) and JC (85%, κ = 0.63), and moderate for IT lesions (75%, κ = 0.42). Agreement for DIS was moderate (80%, κ = 0.58). Discordance between pMRI and cMRI findings was mainly from small PV, JC, and IT lesions that were not depicted on pMRI in 2 of 10 (20%), 2 of 7 (28.6%), and 5 of 8 (62.5%) patients, respectively. The mean size of the largest lesions missed on pMRI was 4.2 mm for PV, 4.5 mm for JC, and 4.4 mm for IT lesions, all of which are smaller than the acquisition slice thickness (5 mm) of the pMRI sequences. Examples of concordant and discordant MRI findings are shown in Figs 1 and 2, respectively.
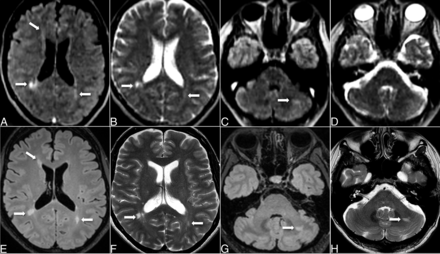
Concordant DIS in 47-year-old woman with optic neuritis. Axial T2-FLAIR (A, C, E, G) and T2-FSE (B, D, F, H) images on 64 mT pMRI (top row, A–D) and 3T cMRI (bottom row, E–H) show multiple supratentorial lesions (A, B, E, F) and a left cerebellar lesion (C, G, H) identified on both 64 mT and 3T. The left cerebellar lesion was seen on the T2-FLAIR (C) but not well depicted on the T2-FSE (D) pMRI sequence.
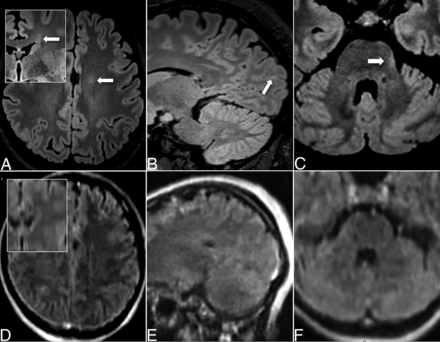
Examples of discordant findings. Axial T2-FLAIR (A, C, D, F) and sagittal T2-FLAIR images (B, E) on cMRI (top row, A–C) and pMRI (bottom row, D–F); insets in (A) and (D) are coronal reconstructions. Small left frontal PV (A, D), left occipital JC (B, E) and left pontine lesions (C, F) were only depicted on cMRI but not pMRI.
Sensitivity and Specificity of pMRI Lesions for DIS
DIS criteria were met on cMRI on 9 of 20 (45%) patients, of whom 5 of 9 (55.6%) were true-positives and 4 of 9 (44.4%) were false-negatives on pMRI. In the 4 patients who were false-negative on pMRI, 2 of 4 (50%) showed PV lesions, but JC and IT lesions were not detected in 2 of 4 (50%) and 3 of 4 (75%) on pMRI, respectively. There were no false-positives for DIS on pMRI.
Test characteristics are summarized in Table 3. The presence of JC and IT lesions as well as fulfilling DIS criteria on pMRI had high specificity and positive predictive values (PPV) (all 100%) for DIS on cMRI. However, sensitivity (SN) was relatively low for these imaging features (44.4% for JC, 33.3% for IT and 55.6% for DIS). PV lesions had high specificity (90.9%), SN (77.8%), and PPV (87.5%) for DIS.
Test characteristics of pMRI findings for DIS on cMRI
Time from Symptom Onset to First MRI
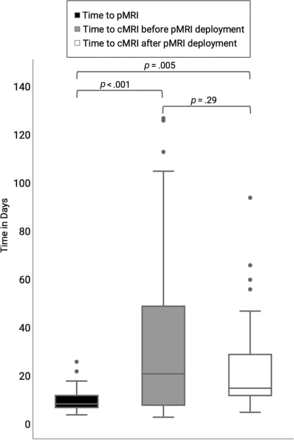
The median time from onset of optic neuritis to imaging was 8.5 (IQR 7−12) days for pMRI, 21 (IQR 8−49) days for cMRI before pMRI deployment, and 15 (IQR 12−29) days for cMRI after pMRI deployment. The Kruskal-Wallis test showed significant time differences between the groups (P = .004). Pair-wise comparison by using the Dunn test showed significantly shorter time from symptom onset to pMRI compared with symptom onset to cMRI before and after pMRI implementation (both P < .01). On the other hand, the time difference from symptom onset to cMRI in those 2 periods was not significantly different (P = .29) (Fig 3).
Time from symptom onset to first MRI.
DISCUSSION
Low-field pMRI is a relatively new technology with growing clinical applications.5,6,11⇓⇓⇓⇓–16,18,19 It offers the advantage of imaging at the point-of-care over cMRI. In this study, we explored the potential clinical impact of pMRI in MS clinical practice to see whether it can provide an earlier point-of-care diagnosis of DIS in patients presenting with optic neuritis, a common early presentation of MS.20 Our results showed moderate concordance of pMRI with cMRI for DIS (80%, κ = 0.58) and that pMRI had high specificity and PPV (both 100%) for DIS. Not surprisingly, pMRI had lower SN (55.6%) for DIS resulting from inability to depict small lesions, particularly IT lesions. Of note, time to pMRI was significantly shorter compared with cMRI because it occurred contemporaneously with the initial assessment in the MS clinic, facilitating an earlier diagnosis of DIS for some patients.
In recent years, the importance of timely diagnosis of MS and early initiation of treatment has become clear, because early treatment leads to better outcomes in pwMS.1,2 MRI is the most important paraclinical tool in establishing an MS diagnosis,3 but access to MRI remains a major cause of delayed diagnosis, particularly in LMICs.4 High cost and infrastructure requirements, as well as the need for highly trained personnel to operate high-field MRI machines limit their availability in resource-limited settings.22,23 Even in Canada, a high-income country where health care is primarily government-funded, wait times for medical treatment and imaging studies have steadily increased in the past several years: in 2023 alone, the average national wait time for a MRI was 12.9 weeks.24 Low-field pMRI may play a role in addressing these barriers.
High-field MRI machines using superconducting magnets typically cost approximately US$ 1 million per Tesla, while low-field machines by using permanent magnets or electromagnets cost only a fraction of this to manufacture. Additionally, low-field MRI systems have lower installation costs and maintenance requirements because they often do not need shielding or cryogenic cooling and consume less energy.22,25,26 These factors make low-field MRI more accessible in LMICs and may augment cMRI in high-income countries. However, literature on the cost-effectiveness of pMRI is limited. A cost-analysis study conducted in a remote area in northern Canada showed potential substantial savings of $854,841 with pMRI deployment compared with transporting patients to a center with cMRI based on an estimation of 50 patients undergoing portable pMRI examinations over 1 year. Over 5 years, total savings were estimated at $7,835,162, assuming a gradual increase in utilization from 50 to 100 patients over that period.8 A recent retrospective semiquantitative descriptive analysis also indicated that implementing pMRI for select neurologic indications in the intensive care unit could potentially replace fixed CT scans in 21% and fixed MRIs in 26.5% of cases, allowing an additional 1676 and 234 patients to undergo fixed CT scans and fixed MRIs, respectively.9
As a proof of concept, Mateen et al17 used a 80 mT MRI to detect white matter lesions in 2 patients with known demyelinating disease in the brain. Arnold et al18 further compared the sensitivity of 64 mT pMRI to 3T cMRI. Lesions as small as 5.7 ± 1.3 mm could be manually detected in 31 of 33 (94%) of pwMS with lesions on 3T MRI. Their study also showed that while lesion conspicuity was similar between the different field strengths, there was significantly more background noise and blurring on low-field images, mainly due to lower spatial resolution.18 Consistent with their results, the largest lesions identified on pMRI and cMRI exhibited similar sizes; but as expected, the smallest lesions detected on cMRI were significantly smaller compared with those on pMRI. Moreover, we found moderate to good concordance of 64 mT pMRI with 3T cMRI for detecting MS lesions and diagnosing DIS, but small lesions were not reliably detected. This aligns with prior studies that also showed that small hemorrhages and infarcts measuring up to 1 cm can be missed on pMRI.6,10⇓–12 The smallest lesions in our study measured 5.3 ± 1.0 mm, comparable to those observed by Arnold et al,18 and approached the spatial resolution of the pMRI scanner. Care must be taken to ensure that lesions smaller than this size are not from volume averaging. Although formal evaluation of quantitative metrics of image quality was not done in this study, most lesions were noticeably more conspicuous on T2-FLAIR compared with T2-FSE sequences, even in the IT brain (Fig 1). This is probably related to the lower TR on the T2-FSE compared with T2-FLAIR (2000 ms versus 4000 ms) on the default parameters set by the manufacturer and decreased CSF pulsation artifacts on pMRI T2-FLAIR images.27 Venous structures also appear hyperintense on pMRI8,18 and should not be mistaken for lesions, particularly superficial cortical veins.
Although lower sensitivity for MS lesions was not surprising, all the patients in this study were expected to undergo a cMRI examination, which is currently the standard of care for MS diagnosis and required for establishing a baseline for future comparison, so missing small MS lesions on pMRI would not have affected patient management. However, one of the objectives of this study was to determine whether pMRI can provide patients with newly diagnosed optic neuritis an expedited point-of-care diagnosis of DIS at the MS clinic before the cMRI. Interestingly, not only meeting DIS criteria, but even having any single PV, JC, or IT lesion on pMRI had high specificity and PPV for definite DIS on cMRI. Moreover, DIS was able to be established at the point of care in 5 of 9 (55.6%) patients. Recent prospective studies suggest that inclusion of the optic nerve as a fifth area for DIS improves the performance of the MS diagnostic criteria,28⇓–30 and this is being considered as a proposed modification for the upcoming revision of McDonald criteria. If the optic nerve were included as another topology in the MS diagnostic criteria, then DIS could also have been diagnosed on an additional 2 of 4 (50%) individuals in our study who were falsely negative for DIS but demonstrated PV lesions on pMRI, enabling a point-of-care identification of DIS in 7 of 9 (77.8%) patients presenting with optic neuritis. Taken together, our findings support a role for pMRI in MS clinical practice.
Although its low magnetic field strength makes pMRI more accessible and enables point-of-care imaging, it also results in decreased image SNR and resolution and results in longer scan times.25 As a result, cMRI remains the standard of care in pwMS. Our study suggests that pMRI when positive is highly specific for DIS, although it does not rule out DIS if negative. This points to a potential complementary role in expediting the diagnosis. Moreover, continued improvement in the device hardware and software, including use of advanced machine learning and super-resolution reconstruction methods,31⇓–33 and possible future use of contrast agents at low-field34,35 may expand the clinical role of pMRI in the future.
Our study has several limitations. First, this was a small, single-center study evaluating only patients presenting with new onset optic neuritis and may therefore be subject to selection bias. Second, we did not perform per lesion analysis, but focused on the practical ability of pMRI to detect MS lesions in the anatomic locations of the brain required to meet the McDonald DIS criteria. Third, DIT could not be assessed as we did not include gadolinium contrast, which in usual doses is not well detected on low-field MRI, although currently an area of active investigation.25,35 Fourth, wait times for cMRI before and after pMRI implementation might have also been affected by institutional workflow changes over time. Fifth, cost-benefit analysis was not performed. Last, workflow and imaging wait times differ among different institutions, sites, and settings that may affect generalizability of these findings, underscoring the importance of context-specific evaluations when considering pMRI application in different health care environments.
CONCLUSIONS
When compared with 3T cMRI, pMRI had moderate concordance, moderate sensitivity, and very high specificity for DIS in patients presenting with new onset optic neuritis. The implementation of pMRI in the MS clinic also reduced the time from symptom onset to a first MRI scan, although whether this is clinically significant remains uncertain. These findings imply that integrating pMRI into clinical practice may complement cMRI, offering more timely neuroimaging at the point of care, which could facilitate early diagnosis. Additional studies are warranted to thoroughly assess the potential role, limitations, and cost-effectiveness of pMRI for both early diagnosis and as an imaging tool in resource-limited settings.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received April 12, 2024.
- Accepted after revision June 22, 2024.
- © 2024 by American Journal of Neuroradiology