Abstract
BACKGROUND AND PURPOSE: Gadolinium leakage in ocular structures (GLOS) is characterized by hyperintense signal in the chambers of the eye on FLAIR and has been reported in association with blood-ocular barrier breakdown in patients with ischemic strokes. The underlying mechanism of GLOS remains poorly understood; however, some studies suggest it may be part of a physiologic excretion pathway of gadolinium. This study aimed to determine the prevalence of GLOS in an unselected patient population.
MATERIALS AND METHODS: A retrospective analysis was conducted on 439 patients who underwent brain MR imaging within 7 days of receiving a gadolinium-based contrast agent injection for a prior MR imaging study. Clinical, imaging, and laboratory data were collected. Descriptive and logistic regression analyses were performed.
RESULTS: GLOS was observed in 26 of 439 patients (6%). The occurrence of GLOS varied with time, with 3 (12%), 14 (54%), 8 (31%), and 1 (4%) patient showing GLOS within 24, 25–72, 73–120, and >120 hours after gadolinium-based contrast agent injection, respectively. Patients with GLOS were older (median age: 72 versus 55 years, P = .001) and had higher median serum creatinine levels (73 versus 64 µmol/L, P = .005) and a lower median estimated glomerular filtration rate (84 versus 101 mL/min/1.73 m2, P < .001). A shorter median time interval between gadolinium-based contrast agent injection and the index brain MR imaging was observed in the group positive for GLOS (62 versus 91 hours, P = .003). Multivariable regression analysis identified the estimated glomerular filtration rate (OR = 0.970; 95% CI, 0.049–0.992; P = .008) and time interval since gadolinium-based contrast agent injection (OR = 0.987; 95% CI, 0.977–0.997; P = .012) as independent factors associated with GLOS.
CONCLUSIONS: GLOS was observed in only a small percentage of patients receiving gadolinium-based contrast agent within 7 days before brain MR imaging. This phenomenon was noted in patients with normal findings on brain MR imaging and those with various CNS pathologies, and it was associated with lower estimated glomerular filtration rates and shorter time intervals after gadolinium-based contrast agent injection. While GLOS may be a physiologic gadolinium-based contrast agent excretion pathway, the presence of ocular disease was not formally evaluated in the included population. Awareness of GLOS is nonetheless useful for appropriate radiologic interpretation.
ABBREVIATIONS:
- eGFR
- estimated glomerular filtration rate
- GBCA
- gadolinium-based contrast agent
- GLOS
- gadolinium leakage in ocular structures
- IQR
- interquartile range
Gadolinium leakage in ocular structures (GLOS) is a recently identified but inadequately understood phenomenon that refers to the enhancement of intraocular fluid compartments and structures following the intravascular administration of IV gadolinium-based contrast agents (GBCAs).1 Although primarily documented in cerebrovascular disorders, such as large-vessel strokes and small-vessel disease,1⇓⇓⇓-5 transient global amnesia,6 and posterior reversible encephalopathy syndrome,7 GLOS has also been observed in various ocular abnormalities affecting both the anterior and posterior eye compartments,8⇓-10 including central retinal artery occlusion11 and optic neuritis.12 These observations have suggested a possible mechanism involving the breakdown of the brain-ocular barrier.
Most interesting, GLOS has also been noted in the anterior eye chamber of healthy infants13 and adults,14 indicating a natural excretion pathway of chelated GBCA via the blood-aqueous barrier as a physiologic event. Other possible physiologic excretion pathways in the eyes include the extraocular glymphatic pathway (through which CSF enters the optic nerve sheath complex), ocular glymphatic system (comprising the aquaporin-4-expressing retinal glial Müller cells in the inner nuclear layer of the retina), and a lymphatic drainage system that ultimately connects to the submandibular nodes.15 Numerous questions surrounding GLOS persist, however, such as its occurrence in a broader spectrum of neurologic disorders, predisposing factors, and implications for ophthalmic health or comorbid conditions.16 Thus, achieving a more complete understanding of this phenomenon is important.
In recent years, a variety of gadolinium-deposition phenomena have been recognized, ranging from asymptomatic gadolinium deposition in patients with normal renal function, termed “gadolinium storage condition,” to the more severe nephrogenic systemic fibrosis at the other end of the spectrum.17,18 These phenomena have had notable consequences for clinicians, patients, and the imaging industry, impacting the medical, medicolegal, and commercial perspectives. Regarding GLOS, however, whether there are short-term or long-term clinical implications associated with its occurrence remains unknown.
Given the immune-privileged status of the eyes and the uncertainty of the consequences of GLOS, the radiologist should recognize and document leakage of GBCA into this protected compartment. The sanctuary status of the eyes is maintained by 2 highly effective blood-ocular barriers, namely the blood-aqueous and blood-retinal barriers. Briefly, the blood-aqueous barrier consists of epithelial and endothelial tight junctions, respectively, in the ciliary body and iris, which are the gatekeepers for the aqueous chamber.19 The blood-retinal barrier has an outer retinal vascular endothelial barrier and an inner retinal pigment epithelial barrier working together to keep unwanted molecules out of the vitreous chamber.20 Why, how, and when the blood-ocular barriers become permeable to GBCA remains a focus of contemporary research.
The time window for the onset of GLOS continues to evolve. Before Deike-Hofmann et al14 explored the pathway of GBCA through the glymphatic system up to 24 hours postinjection, there was little interest in extending the window beyond the usual 10 minutes postinjection. Other authors exploring GLOS extended the observation window up to 72 hours postinjection in prospective studies.1,5 The possibility of GLOS occurring after 72 hours has not yet been explored.
The primary objective of this study was to investigate the prevalence of GLOS in routine brain MR imaging performed within 7 days after prior gadolinium-enhanced MR imaging on a heterogeneous population of patients encountered in routine neuroradiology practice and to identify factors associated with the occurrence of GLOS. We also aimed to explore the possibility of GLOS as a potential physiologic process, further contributing to the understanding of this intriguing phenomenon.
MATERIALS AND METHODS
This study was approved by the institutional research ethics board of St. Michael's Hospital, Unity Health Toronto. The patient consent form was waived.
Patient Population
The MR imaging report database was used to retrospectively identify patients who had undergone brain MR imaging within 0–168 hours (7 days) of any type of GBCA injection for any contrast-enhanced MR imaging procedure (regardless of body part) between January 1, 2011, and December 31, 2020. Patients were excluded if there were severe artifacts in the orbits on brain MR images, no available axial FLAIR sequence, ocular blood products present on the index brain MR imaging study (if the baseline study was of the brain) as evidenced by abnormal susceptibility on SWI or intrinsic high signal on T1-weighted images, or incomplete laboratory data. The FLAIR sequence with fat saturation was excluded from the analysis because of frequent incomplete fluid signal suppression in the orbits and its proneness to motion-related artifacts.
Demographic and clinical data were extracted from the electronic medical records, including age and sex, indications for brain MR imaging, prior cataract surgery, serum creatinine level at the time of index brain MR imaging, and the corresponding estimated glomerular filtration rate (eGFR) calculated using an online calculator on the basis of the National Kidney Foundation and the American Society of Nephrology Task Force recommendations.21
MR Imaging Protocol
All patients had at least 1 initial gadolinium-enhanced MR imaging, regardless of the body part or clinical indications, performed following IV administration of any of the following GBCAs: gadobenate dimeglumine (MultiHance, 529 mg/mL; Bracco); gadoteriol (ProHance, 279.3 mg/mL; Bracco); and gadobutrol (Gadovist, 604 mg/mL; Bayer) at a dose of 0.1 mL/kg body weight.
Within 0–168 hours after the initial gadolinium-enhanced MR imaging, the patients included in the review underwent an index brain MR imaging performed on a 1.5T MR imaging scanner (Achieva DS; Philips Healthcare) using a 6-channel head coil. The axial 2D FLAIR sequences in the index brain MR imaging were acquired either without GBCA or concurrent with/immediately after GBCA injection before the acquisition of the postgadolinium T1-weighted sequence (if the index brain MR imaging protocol included a GBCA injection, as per our institution's protocol). All included axial 2D FLAIR images were acquired without fat saturation with the following sequence parameters: TR/TE/TI/flip angle = 11,000 ms/140 ms/2800 ms/90°, section thickness = 5 mm, section spacing = 6 mm, number of excitations = 1, matrix size = 240 × 240, FOV = 200 mm.
The dose and type of GBCAs injected on the initial gadolinium-enhanced MR images and the time interval between the prior gadolinium-enhanced MR imaging scan and the index brain MR imaging were recorded.
Image Analysis
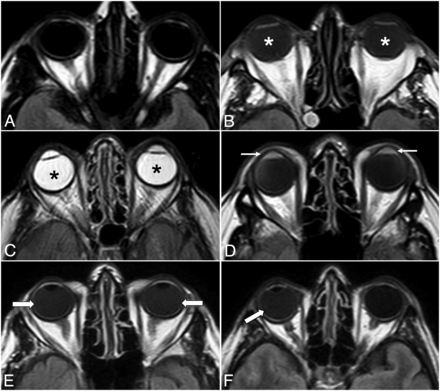
The MR imaging examinations of the patients who fulfilled all eligibility criteria were electronically anonymized and placed in a designated folder on the institutional PACS. Two readers (R.O. and T.R.L.) independently reviewed the images in a random order, blinded to the antecedent imaging and clinical and laboratory data of the patients. If there was discordance between the 2 readers, a third senior reviewer (S.S.) assessed the findings and a consensus was reached. The presence or absence of GLOS, unilateral or bilateral ocular involvement, and involvement of the aqueous and/or vitreous chamber on the index brain MR imaging scan were documented. GLOS was defined as a lack of or incomplete suppression of fluid signal in the fluid-containing structures of the globes on the axial FLAIR sequence without fat saturation. Specifically, findings were considered mildly positive for GLOS if ocular fluid showed at least a higher signal intensity relative to the normal CSF but less than that of normal white matter on the same section; and they were intensely positive for GLOS if the signal intensity was greater than that of the normal white matter, approximating or equivalent to that of unsuppressed fat signal on the same section (Fig 1). The studies were visually inspected for GLOS to simulate routine clinical practice in this observational, qualitative study. Subsequently, post hoc analyses used ROIs to confirm that the signal was indeed higher than that of normal CSF or normal white matter for mild and intense GLOS, respectively.
Illustrative examples of different GLOS patterns. Axial FLAIR images show normal globes (A), mild bilateral GLOS (asterisks in B), strong bilateral GLOS (asterisks in C), GLOS in the aqueous (arrows in D) and vitreous chambers (arrows in E), and unilateral GLOS involving the right globe (arrow in F).
Statistical Analysis
The baseline and demographic characteristics were summarized using descriptive statistics and compared using a χ2 or Fisher exact test for categoric variables and Mann-Whitney U tests for continuous variables. The Kolmogorov-Smirnov test of normality showed that data were not normally distributed with P < .05. Univariable and multivariable logistic regression analyses were performed to determine which factors were associated with the presence of GLOS. The variables of interest from the univariable analyses (age, sex, eGFR, time interval since GBCA injection, and the presence of prior cataract surgery) were included in the multivariable model using the enter method. A P value < .05 was considered statistically significant. Data were analyzed using SPSS Statistics for Windows, Version 25 (IBM).
RESULTS
A total of 439 patients (242 women [55.1%]; median age, 55 years; interquartile range [IQR], 45–67 years) fulfilled all inclusion criteria. Clinical indications for MR imaging included brain tumors (n = 150, 34.2%), intracranial hemorrhage (n = 29, 6.6%), ischemic stroke (n = 24, 5.5%), baseline assessment post-endovascular treatment (n = 83, 18.9%), infection/inflammation (n = 39, 8.9%), and others (n = 81, 18.5%). Thirteen (3%) patients had prior gadobutrol injection; 270 (61.5%), gadobenate dimeglumine; 152 (34.6%), gadoteridol; and 4 (0.9%) had injections using 2 different GBCAs. The index brain MR imaging was acquired without GBCA in 260 (59.2%) and was concurrent with GBCA injection in 179 (40.8%) patients. In terms of renal function, 320 (72.9%) patients were classified as G1, 102 (23.2%) as G2, and only 17 (3.9%) as G3 (Online Supplemental Data).
GLOS Prevalence and Spatiotemporal Distribution
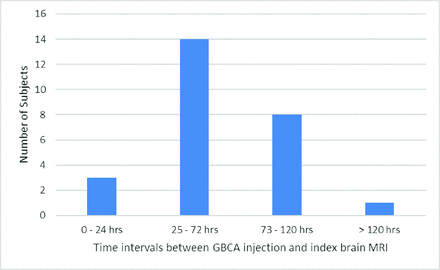
There was imaging evidence of GLOS in 26/439 (5.9%) patients (14 women [53.8%]; median age, 71.5 years; IQR = 51.0–77.5 years). Among the patients with GLOS (n = 26), the index MR imaging was acquired without GBCA in 19 (73.1%) and concurrent with GBCA injection in 7 (26.9%). MR imaging showed brain tumors in 9 (34.6%), normal brain findings in 4 (15.4%), acute infarcts in 2 (7.7%), and hemorrhage in 2 (7.7%) (Online Supplemental Data). GLOS was bilateral in 22 (84.6%) and unilateral in 4 (15.4%). It involved the aqueous chamber only in 1 (3.8%), the vitreous chamber only in 18 (69.2%), and both aqueous and vitreous chambers in 7 (26.9%). GLOS was mildly positive in 21 (80.8%) and intensely positive in 5 instances (19.2%). Of the patients with intensely-positive GLOS, all 5 cases (100%) involved the aqueous chamber, while 2 cases (40%) involved both the aqueous and vitreous chambers (Table 1 and the Online Supplemental Data). GLOS was seen in 3 (11.5%), 14 (53.8%), 8 (30.8%), and 1 (3.8%) patients who underwent the index brain MR imaging within 24 hours, 25–72 hours, 73–120 hours, and >120 hours of the prior gadolinium-enhanced MR imaging, respectively (Fig 2).
Bar chart showing the number of subjects positive for GLOS at different time intervals between the administration of gadolinium-based contrast agent and the index brain MR imaging.
Spatiotemporal distribution of GLOS
Comparison of Patients with and without GLOS
Patients with GLOS were older (median age, 71.5 [IQR = 51–75.5] versus 55 [IQR = 44–66] years, P = .001), had higher median serum creatinine levels (72.5 [IQR = 67.8–98] versus 64 [IQR = 44–77] µmol/L, P = .005), a lower median eGFR (84 [IQR = 61.5–99.5] versus 101 [IQR = 89–113] mL/min/1.73 m2, P < .001), and a shorter median time interval between the injection of GBCA and the index brain MR imaging (62 [IQR = 35–85.3] versus 91 [IQR = 51.5–133] hours, P = .003) compared with those without GLOS. The median dose of GBCA (16 [IQR = 10–26.5] versus 16 [IQR = 10–20] mL, P = .9) was not significantly different between the 2 groups. There was no association between GLOS and the type of GBCAs used (P = .9, Fisher exact test) (Online Supplemental Data).
Predictors of GLOS
Univariable logistic regression showed that GLOS was significantly associated with older age (OR = 1.051; 95% CI, 1.020–1.083; P = .001), higher serum creatinine levels (OR = 1.026; 95% CI, 1.010–1.041; P = .001), a lower eGFR (OR = 0.961; 95% CI, 0.943–0.979; P = <.001), a shorter time interval between the GBCA injection and the index brain MR imaging (OR = 0.986; 95% CI, 0.976–0.996; P = .005), and the presence of prior cataract surgery (OR = 3.744; 95% CI, 1.477–9.490; P = .005). Multivariable analysis showed that only a lower eGFR (OR = 0.970; 95% CI, 0.049–0.992; P = .008) and a shorter time interval between GBCA injection and the index brain MR imaging (OR = 0.987; 95% CI, 0.977–0.997; P = .012) were found to be statistically significant independent predictors of GLOS; however, the magnitude of OR is small (Table 2).
Associations of selected features with occurrence of GLOS
DISCUSSION
In our study, GLOS was demonstrated on MR imaging in 5.9% of patients imaged for a broad range of clinical indications. Its occurrence was not limited to patients with neurovascular conditions but was also seen in those with other various pathologies and even in patients without any identified abnormality of the brain and orbits on MR imaging. This expanded distribution of GLOS underscores the importance of considering it as a potential physiologic phenomenon in a diverse cohort of patients undergoing gadolinium-enhanced MR imaging.
Our findings showed that most (around 64%) GLOS occurred within 72 hours of prior IV GBCA administration, while 36% of GLOS occurred after the usual 72-hour time window. Univariable regression analysis revealed associations between GLOS and older age, lower eGFR, prior cataract surgery, and a shorter time interval from the initial gadolinium-enhanced MR imaging scan to the index brain MR imaging. In multivariable regression analysis, however, only lower eGFR levels and a shorter time interval from prior GBCA injection to the index brain MR imaging were identified as statistically significant independent predictors of GLOS, though the effect sizes were relatively small. The dose, frequency, and type of GBCAs administered had no impact on the occurrence of GLOS.
Distinct from the general diffusion of GBCAs into extravascular spaces seen commonly in patients with chronic renal disease,22 GLOS was recently recognized in a cohort of patients with stroke at the US National Institutes of Health.1 Subsequent work on GLOS by other authors has mainly focused on strokes and TIAs, leading to the prevailing perception of GLOS as a manifestation of ischemic insult.1⇓⇓⇓⇓⇓-7 However, a recent study from a stroke unit showed that the occurrence of GLOS is not specific to stroke but rather possibly represents blood-retinal barrier dysfunction related to small-vessel disease.5 In our study, which included a heterogeneous group of different neurologic conditions not limited to patients with stroke, we observed the occurrence of GLOS in patients with other entities such as brain tumors, intracranial hemorrhage, infectious/inflammatory processes, post-intracranial aneurysm coiling, and even in those with normal brain MR imaging findings. Our findings are consistent with those in prior studies showing an association between GLOS and older age.1,3,5 Additionally, we observed an association with lower eGFR, similar to findings of Galmiche at al,5 but we did not find any association with the dose of GBCA administered.
The 5.9% prevalence of GLOS in our study is much lower than the previously reported rates of 30%–76% in the setting of acute-onset permanent or reversible brain ischemia.1⇓-3,5⇓-7 Galmiche et al5 also sought to understand GLOS beyond stroke, reporting a prevalence of approximately 30% in their study subjects with proved ischemic stroke or TIA. In contrast, only 5.5% of our study subjects had ischemic stroke or TIA. Several factors may have contributed to our lower GLOS prevalence, including the extension of the time interval between the GBCA injection and the index brain MR imaging beyond 72 hours, the younger age of our patients, and fewer patients with impaired renal function. Other authors who reported a higher prevalence of GLOS reviewed MR imaging performed only within 24–72 hours after GBCA injection.1,5 In our study, most documented GLOS (14/26, 53.8%) also manifested between 24 and 72 hours after GBCA administration. Furthermore, only 3.9% of our subjects had an eGFR between 30 and 60 mL/min/1.73 m2, and none had an eGFR <30 mL/min/1.73 m2, representing better renal function than found in the population included in previous studies. The interplay of a more heterogeneous, relatively younger, healthier population, at least from the renal function perspective, likely resulted in the low prevalence of GLOS in our study.
On the basis of our observations, we hypothesize that GLOS might represent the physiologic excretion of GBCAs into the ocular globes via the blood-aqueous barrier, with accumulation in the vitreous chamber, which was captured at a specific time delay after GBCA injection. A recent study using delayed heavily T2-weighted and fat-suppressed FLAIR at 3 and 24 hours post-GBCA injection demonstrated physiologic excretion of GBCA into the aqueous chamber of the eye via the ciliary body even in patients without blood-brain barrier disruption or renal impairment. Excreted GBCA was observed to migrate from the aqueous chambers and accumulate in the vitreous chambers.14 Similar increased signal intensity in the aqueous chamber after GBCA administration with subsequent migration to the vitreous chamber has also been shown in healthy infantile eyes and healthy controls.10,13 Most interesting, in previous studies evaluating GLOS in patients with stroke, isolated aqueous chamber involvement was more commonly seen on scans with shorter time intervals between the initial and follow-up MR imaging,1,2 possibly, to some extent, also related to this physiologic pathway. We found evidence of GLOS in 4/26 (15.4%) patients with normal MR imaging findings of the brain and orbits, only one of whom had impaired renal function. In addition, while GLOS was significantly associated with a lower eGFR in our study, many patients positive for GLOS on MR imaging did not have marked renal impairment. These findings indicate that GLOS may be a form of physiologic distribution or excretion of GBCA in the eye, particularly in the absence of any identifiable medical cause to explain its occurrences.
Although prior cataract surgery and age were found to be statistically associated with the presence of GLOS on univariable regression analysis, no statistical significance was noted when controlling for eGFR levels. While ocular enhancement on the postcontrast FLAIR sequence after ocular surgery has been previously demonstrated23 and it is known that blood-ocular barrier dysregulation may persist even several years after cataract surgery,24,25 there has been no previous study showing a direct association of prior cataract surgery with GLOS, to the best of our knowledge. In addition, several studies have also demonstrated GLOS in various ophthalmologic pathologies involving the anterior and posterior eye compartments, including optic neuritis.9⇓⇓-12,23 Therefore, locoregional alterations in the structural and functional integrity of the blood-aqueous and blood-retinal barriers from ocular diseases and postsurgical causes should be considered in the differential diagnosis and overall assessment of GLOS.
Our study has 3 main areas of strength. First, our retrospective study is one of the largest clinical investigations on GLOS and is also among the few conducted on a heterogeneous group of patients not limited to those having neurovascular conditions, representing a real-world clinical setting. Second, we also report 2 potential independent predictors of GLOS (lower renal functional status and short time interval from GBCA injection to index brain MR imaging). Last, we report the occurrence of GLOS beyond the previously reported usual 72-hour time window post-GBCA administration.1,5 Delayed physiologic excretion might account for the occurrence of GLOS later during the first week in more than one-third of the patients who were positive for GLOS.
Our study has some limitations as well. First, this study is a single-center retrospective observational study in a heterogeneous population with a relatively small number of patients with GLOS. Larger prospective studies are needed to validate our findings and gain a more comprehensive understanding of the GLOS incidence and risk factors and to evaluate the impact of ophthalmologic disease on GLOS. On the basis of our study, the association between ocular diseases and the occurrence of GLOS also cannot be determined. Next, exclusion of patients with an eGFR of <30 mL/min/1.73m2 as per our institutional protocol during the period of data collection precludes evaluation of this cohort. Regarding our available index MR imaging studies, when performed with gadolinium, they included a 2D FLAIR sequence acquired concurrently with GBCA injection, which could potentially affect our results. While GLOS has been found to occur as early as 12–20 minutes post-GBCA injection, however, this occurrence was documented in infantile eyes with immature blood-aqueous barriers or with a heavily T2-weighted FLAIR sequence, which is more sensitive to GBCA compared with a routine 2D FLAIR sequence.10 In addition, our imaging studies were performed at 1.5T, did not have fat suppression on FLAIR imaging, and used 2D FLAIR sequences with 5-mm section thicknesses, which could limit our ability to detect GLOS. Finally, our retrospective data derived from a routine MR imaging database precludes direct comparison with prospective data obtained with dedicated orbit surface coils and/or a heavily T2-weighted FLAIR sequence, and our rate of subjects positive for GLOS might have been understated. Despite these limitations, our study contributes data regarding the prevalence, predictors, and distribution of GLOS in patients undergoing brain MR imaging for various clinical indications.
CONCLUSIONS
Our study found GLOS in a small percentage of brain MR imaging studies in an unselected population of patients who received GBCA injections within 7 days before the index brain MR imaging. GLOS may result from the physiologic excretion of GBCAs, and its occurrence is not limited to neurovascular conditions. Lower eGFR and a shorter time interval between GBCA injection and the index brain MR imaging were found to be independently associated with GLOS. Additional research is needed to further understand the mechanism and assess the clinical significance of GLOS. Awareness of GLOS would be useful for appropriate radiologic interpretation and patient counseling when encountering this phenomenon in routine clinical practice.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received July 28, 2023.
- Accepted after revision October 25, 2023.
- © 2024 by American Journal of Neuroradiology