Abstract
BACKGROUND AND PURPOSE: DWI-detected ischemic lesions are potential complications of endovascular procedures that are performed to treat intracranial aneurysms. We completed a systematic review and meta-analysis to identify the occurrence of DWI-detected ischemic lesions after endovascular treatment for intracranial aneurysms.
MATERIALS AND METHODS: A systematic literature search of PubMed, the Web of Science, EMBASE, and Scopus between January 2000 and June 2022 of post-endovascular procedures for intracranial aneurysm studies was conducted using the Nested Knowledge AutoLit software. The main outcome was DWI-detected ischemic lesions within 5 days of the procedures. Information regarding associated risk factors such as the type of procedure, patient demographics, and aneurysm characteristics was also collected.
RESULTS: Twenty-nine studies with 2686 patients were included. The overall incidence of DWI ischemic lesions was 47.0% (95% CI, 39.6%–55.8%). The highest rate of lesions was seen with flow diversion at 62.4% (95% CI, 48.4%–80.5%), followed by complex procedures at 49.3% (95% CI, 29.5%–82.1%), stent-assisted coiling at 47.5% (95% CI, 34.6%–65.3%), simple coiling at 47.1% (95% CI, 35.7%–62.3%), and balloon-assisted coiling at 37.0% (95% CI, 28.3%–48.4%). The differences among different techniques were not statistically significant; however, there was significant heterogeneity and a significant risk of publication bias among included studies.
CONCLUSIONS: Many patients who undergo endovascular procedures for intracranial aneurysms present with new postprocedural DWI-detected ischemic lesions, regardless of the endovascular procedure used. Future studies and meta-analyses are needed to investigate early and long-term outcomes of such small infarcts.
ABBREVIATION:
- APC
- annual percentage change
Endovascular neurointerventional procedures have been used since the 1990s with the development of different techniques that obviate the need for a craniotomy. Endovascular techniques include simple coiling, stent-assisted coiling, balloon-assisted coiling, and flow diversion. Although less invasive than traditional surgery, these techniques have been associated with periprocedural complications ranging from aneurysmal rupture to different thromboembolism presentations. DWI-identified ischemic lesions after endovascular procedures have been reported to be a common occurrence with unknown clinical significance.1⇓⇓-4
The overall incidence of DWI-positive lesions previously reported after endovascular procedures was 1 in 2 patients; however, previous literature was based on outdated studies, older devices and techniques.1 We propose that the rates of DWI-detected lesions following endovascular procedures may vary with the introduction of new endovascular techniques and procedures. To determine the overall incidence of perioperative infarctions on DWI in patients undergoing endovascular treatment for intracranial aneurysms, we performed a systematic review and meta-analysis.
MATERIALS AND METHODS
Literature Search and Inclusion Criteria
In-depth article reviews were performed by A.B.H. and S.G. using keywords such “flow diverter,” “coiling,” “pipeline,” “endovascular,” “DWI-MR imaging,” “diffusion,” “restricted diffusion,” “MR imaging,” “diffusion-weighted imaging,” “cerebral aneurysm,” and “intracranial aneurysm.” During the review process, the last set of Boolean traits found in the Online Supplemental Data were applied. On June 10, 2022, this search algorithm was used to search PubMed, the Web of Science, EMBASE, and Scopus.
The inclusion criteria were observational studies or randomized controlled trials between January 2000 and June 2022 that consisted of at least 10 consecutive patients treated for intracranial aneurysms by endovascular means with endovascular coiling or flow diversion and DWI examinations performed within 5 days of endovascular treatment in all patients. In addition, the reference lists from included studies were retrieved for possible inclusion of missed publications. Exclusion criteria were studies that had <10 patients, reported outcomes of parent artery occlusion, included only symptomatic patients who underwent postoperative DWI, used intrasaccular devices (such as the Woven EndoBridge [WEB]; Sequent Medical), and conference proceedings. In addition, studies that did not provide the number of patients who had no lesions on DWI and studies for which an English translation was not available were excluded.
The current study used a novel semiautomated software platform (AutoLit, Nested Knowledge; https://wiki.nested-knowledge.com/doku.php?id=wiki:autolit) for screening of studies and extraction of data. Two independent reviewers performed the screening, and a third reviewer arbitrated disagreements. Baseline characteristics of patients and aneurysm types such as aneurismal size, location, and rupture status were extracted. During data extraction, whenever >2 means for subgroups were available, the formula found in the Online Supplemental Data from the Cochrane Guide for Systematic Reviews and Metanalysis was used.5 The guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) were followed, as well.
Outcomes and Patient Groups
DWI-detected ischemic lesions within 5 days of the endovascular procedure were the primary end point. Treatment groups were stratified by the type of procedure: simple coiling, stent-assisted coiling, balloon-assisted coiling, and flow diversion. When ≥2 procedures and multiple catheters were used, it was termed a complex procedure. Patient sex (male versus female), aneurysm size (considered small if <10 mm, giant if >10 mm),6 location (anterior or posterior circulation), and rupture status (ruptured or unruptured) were dichotomized.
Risk of Bias Assessment
We selected items from the Newcastle-Ottawa Quality Assessment Scale to fit the type of included studies. In 3 domains with 8 criteria and a maximum score of 8, we queried the following study characteristics using a previous study by one of the authors: 1) patient groups clearly defined (1 point given if patient groups were clearly defined by the type of endovascular procedure, zero points given if not); 2) outcomes reported (1 point for reports taken from hospital data, 2 points for reports by radiologists for the study using DWI); 3) outcomes reported for each patient group studied (1 point given if an outcome was reported for each procedure, zero points given if not); 4) imaging interpreted by an independent reader or interpreted by the operator (1 point if independent readers read it, zero points given if not); 5) readers blinded to the clinical status of the patient (1 point given if blinded, zero points given if not); 6) multiple readers used and interobserver agreement assessed (1 point given if interobserver agreement was assessed, zero points given if not); and 7) the study followed a specific study protocol in which all patients underwent MR imaging at the same time point (1 point given if the timing of the postprocedural MR imaging was provided, zero points given if not).1
Statistical Analysis
The cumulative incidence rates with corresponding 95% CIs were calculated using R statistical and computing software, Version 4.2.2 (http://www.r-project.org/) and the package ‘meta’ (https://cran.r-project.org/web//packages/meta/meta.pdf). A random-effects model was used to pool the data due to methodologic heterogeneity among the included studies, and subgroup analysis was performed to obtain the estimate per year. Heterogeneity was assessed using the Cochran Q and I2 tests. P values < .05 for the Q statistic indicated statistical significance. An I2 of >50% suggested moderate-to-high heterogeneity. Whenever there were ≥10 studies included, the Egger regression test was used to assess publication bias. If publication bias existed, we used the trim-and-fill method to adjust for funnel plot asymmetry and calculate the bias-adjusted estimates.7,8
By means of the Joinpoint Regression Trend Analysis Software, Version 4.9.1.0 (https://surveillance.cancer.gov/joinpoint/), the pooled cumulative incidence rates and their standard errors were used to conduct a joinpoint regression analysis to explore any trends across the years.9,10 This was conducted to calculate the average annual percentage change and annual percentage change (APC), selecting the best-fitting piecewise continuous log-linear model. The minimum number of “joinpoints” required to fit the data was determined using a permutation test.11,12 The weighted Bayesian Information Criterion was the model adopted, and the empirical quantile methods were used to estimate confidence intervals.13 The empirical method does not produce a test statistic or P value.13
RESULTS
Search Results and Patient Population
The literature search retrieved 491 studies, from which 209 duplicate studies were removed. From the remaining 282 studies, title and abstract screening resulted in 160 studies being excluded. Of the 122 studies remaining, full-text screening resulted in another 93 studies being excluded, as illustrated in Fig 1, which shows the process of identifying studies through databases and registers.
Identification of studies via databases and registers.
Risk of Bias
Of the 29 studies included in this meta-analysis, 2 had a very high risk of bias with scores between 0 and 3 points, 22 had a high risk of bias with scores of 4–6 points, and 5 had a low risk of bias scoring 7–8 points, as determined by the Modified Newcastle Ottawa Scale.14
Characteristics of the Included Studies
Twenty-nine studies with a total of 2686 patients presenting with 3687 intracranial aneurysms fulfilled our inclusion criteria and composed our analysis.15⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-43 Endovascular procedures were simple coiling in 1184 patients, stent-assisted coiling in 662 patients, balloon-assisted coiling in 328 patients, flow diversion in 211 patients, and complex procedures in 301 patients. From studies that reported aneurysm rupture status, 275 were ruptured during treatment, while 2552 were unruptured. Among the studies that reported the location of the aneurysms, 1624 were in the anterior circulation and 510 were in the posterior circulation.
Study Outcomes
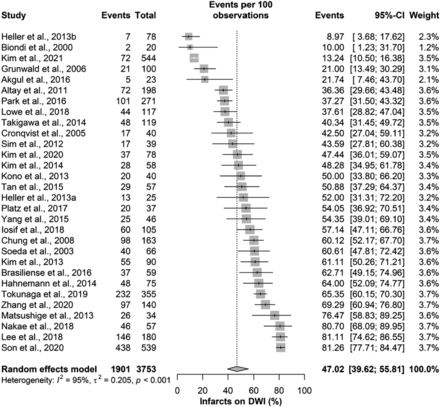
The overall incidence of DWI lesions was 47.0% (95% CI, 39.6%–55.8%), with significant heterogeneity among the included studies (I2 = 95%; P value < .001; Fig 2). Moreover, there was a significant risk of publication bias as assessed with the Egger regression test (P value < .001; Fig 3).
Infarcts on DWI.
Publication bias as assessed with the Egger regression test.
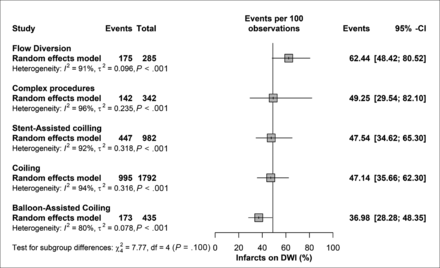
On further subgrouping based on the treatment technique used, the highest rate of lesions was seen with flow diversion at 62.4% (95% CI, 48.4%–80.5%), complex procedures at 49.3% (95% CI, 29.5%–82.1%), stent-assisted coiling at 47.5% (95% CI, 34.6%–65.3%), simple coiling at 47.3% (95% CI, 35.5%–63.7%), and balloon-assisted coiling at 35.9% (95% CI, 25.5%–50.6%). The differences among different techniques were not statistically significant (P value = .139), with significant heterogeneity among the included studies in all subgroups (Fig 4).
The subgroup of treatment technique used and DWI lesions.
For trend analysis, there was a significant reduction in DWI lesions through the full range of years from 2000 to 2022, with an average annual percent change of −5.0 (95% CI, −7.6 to −1.9). Moreover, there was 1 joinpoint identified in 2019, with a significant reduction in detected ischemic lesions (APC = −50.0; 95% CI, −63.1 to −35.0) after a relative stability in rates before that (APC = 1.6; 95% CI, −0.2−5.0; Online Supplemental Data).
Impact of Aneurysm Characteristics and Sex
Aneurysms were more likely to be in the anterior circulation compared with the posterior circulation in DWI-detected lesions (OR = 8.56; 95% CI, 2.97–24.7, P value < .001). Men were less likely than women to have aneurysms with DWI-detected lesions (OR = 0.15; 95% CI, 0.1–0.22, P value < .001). Large-sized aneurysms were less common than small-sized aneurysms (OR = 0.05; 95% CI, 0.02–0.16, P value < .001), while rupture status was comparable (OR = 0.25; 95% CI, 0.02–2.2, P value = 0.21). For all aneurysm characteristics and sex of patients, there was significant heterogeneity among the included studies (Fig 5).
Risk factors and DWI lesions.
DISCUSSION
Our systematic review and meta-analysis found that the incidence of DWI-positive lesions after endovascular procedures for intracranial aneurysms was 47%. We found no statistically significant difference among techniques. These results highlight the high incidence of DWI-positive lesions, which are statistically similar among different endovascular treatments.
Previous studies have examined the clinical impact of DWI-detected lesions. Almost half of these lesions are asymptomatic, and ischemic lesions have been shown to regress if their size is small (<5 mm), resulting in favorable rates of good clinical outcomes.44 Studies are needed to further validate these findings using clinical outcome measures such as a standardized method of assessing the clinical relevance of lesions like the mRS. Other literature has evaluated the prevention of this postprocedural complication with remote ischemic preconditioning, which has been shown to be safe and effective.45
We found that the rate of silent infarcts in patients treated with flow diverters was higher than in those treated with coils alone, though this was not statistically significant. Flow diversion leads to more thromboembolic complications than coiling alone, despite the inherent thrombogenic properties of coils and flow diverters. Because flow diverters are high density and have large endoluminal surfaces, they have a high thrombosis risk in the parent arteries. An embolized thrombus can occur from the shearing stress caused by blood flowing through the device. Because lower density coils are placed in the aneurysm sac outside the cerebral circulation, thrombi forming on them are less likely to embolize.1
Studies have shown that DWI-positive lesions are not significantly different between ruptured and unruptured aneurysms (as shown in Fig 5), are associated with long procedural times, and decrease in incidence with postprocedural anticoagulation, all of which are consistent with the results of our present analysis.46 Additionally, previous literature has demonstrated that the presence or number of ischemic lesions on DWI is not related to cognitive changes following coil embolization.47 Periprocedural thromboembolic events identified on DWI have been shown to be reduced with a dual-antiplatelet agent (for unruptured aneurysms), and patients are given preoperative low-molecular-weight heparin to prevent this complication.48
We could not identify a discernible trend in the incidence of DWI lesions with time. The absence of a notable temporal trend suggests that the occurrence of DWI lesions did not change before and after 2017 (Online Supplemental Data).
Our study has limitations. We included retrospective, nonrandomized studies, and studies were inconsistent in their reporting of results. We did not consider the use of anticoagulants and individual antiplatelet therapy. We could not account for the differences among institutions performing endovascular procedures, the experience of the interventionalists who performed the procedures, and the radiologists who read the DWIs. There were associations between baseline characteristics such as female sex, anterior aneurysm, small aneurysm size, and the incidence of postprocedural DWI lesions. The studies included did not consistently report atherosclerotic risk factors, use of anticoagulants, and other aneurysmal characteristics.32 Finally, we did not have access to individual patient data, limiting the data that could be analyzed. In our study, the presence or absence of DWI lesions was used as the primary criterion for determining their occurrence. However, we acknowledge that a more nuanced approach, considering the size, number, and distribution of DWI lesions, could provide a more comprehensive understanding of the ischemic risk and its clinical implications. Because these details are currently lacking in the literature, further studies are needed to address these concerns.
CONCLUSIONS
In this systematic review and meta-analysis of 2686 patients, the rate of DWI-detected ischemic lesions was 47.0% (95% CI, 39.6%–55.8%). Rates of lesions were statistically similar among all included endovascular treatments. Further prospective and randomized studies are needed to elucidate the DWI-detected ischemic lesion rate after endovascular procedures and their lasting complications.
ACKNOWLEDGMENTS
The authors acknowledge Karl Holub, Stephen Mead, Jeffrey Johnson, and Darian Lehmann-Plantenberg for their design, development, and support of the Nested Knowledge meta-analytical software. The authors thank Desiree Lanzino, PhD, and Sonia Watson for their assistance in editing the manuscript.
Footnotes
Research reported in this publication was supported, in part, by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award No. R01NS076491.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received February 23, 2023.
- Accepted after revision September 8, 2023.
- © 2023 by American Journal of Neuroradiology