Abstract
BACKGROUND AND PURPOSE: Net water uptake is qualified as an imaging marker of brain edema. We aimed to investigate the ability of net water uptake to predict 90-day functional outcome in patients with acute ischemic stroke and large-vessel occlusion.
MATERIALS AND METHODS: A total of 295 consecutive patients were retrospectively enrolled. Automated ASPECTS–net water uptake was calculated on the admission CT. The relationship between ASPECTS–net water uptake and 90-day neurologic outcome was assessed. The independent predictors of favorable outcome (mRS score ≤2) were assessed using multivariate logistic regression analysis and receiver operating characteristic curves and stratified by the ASPECTS.
RESULTS: Favorable 90-day outcomes were observed in 156 (52.9%) patients. ASPECTS–net water uptake (OR, 0.79; 95% CI, 0.70–0.90), NIHSS scores (OR, 0.91; 95% CI, 0.87–0.96), age (OR, 0.96; 95% CI, 0.94–0.99), and vessel recanalization (OR, 7.78; 95% CI, 3.96–15.29) were independently associated with favorable outcomes at 90 days (all, P < .01). A lower ASPECTS–net water uptake independently predicted a good prognosis, even in the subgroup of patients with low ASPECTS (≤5) (P < .05). An outcome-prediction model based on these variables yielded an area under the receiver operating characteristic curve of 0.856 (95% CI, 0.814–0.899; sensitivity, 76.3%; specificity, 81.3%).
CONCLUSIONS: ASPECTS–net water uptake could independently predict 90-day neurologic outcomes in patients with acute ischemic stroke and large-vessel occlusion. Integrating ASPECTS–net water uptake with clinical models could improve the efficiency of outcome stratification.
ABBREVIATIONS:
- AIS
- acute ischemic stroke
- AUC
- area under the curve
- IQR
- interquartile range
- LVO
- large-vessel occlusion
- MT
- mechanical thrombectomy
- mTICI
- modified TICI
- NWU
- net water uptake
- ROC
- receiver operating characteristic
With the development of tPA and mechanical thrombectomy (MT), there has been a remarkable improvement in the functional outcome of patients with acute ischemic stroke (AIS). In particular, the recent successes of the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention with Trevo (DAWN) and Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE-3) trails have demonstrated the benefit of MT for patients with AIS with large-vessel occlusion (LVO) who presented within 6–24 hours after symptom onset.1,2 The trials used strict eligibility criteria based on advanced imaging with CTP or MR imaging for patient selection.3 However, perfusion imaging is not widely available in emergency settings due to technical and software requirements. Moreover, vendor software, institutional methods, and thresholds vary widely, causing a controversy over stroke imaging.4,5 The use of fixed thresholds to distinguish core and penumbra for the processing of perfusion imaging is not optimal because tissue viability after AIS is time-dependent and has substantial intrasubject variability.6,7
Recent studies found that NCCT yielded no significant difference in clinical and safety outcomes compared with CTP or MR imaging for selecting eligible patients.8⇓-10 NCCT was considered potentially more sensitive than relative CBF for the detection of irreversible injury in the extended time window.11 Net water uptake (NWU) is a recently introduced NCCT-based parameter that can be used as a pathophysiologic imaging marker of brain edema and an individualized indicator of “tissue clock” in patients with AIS.12 An early elevated level of NWU in ischemic lesions was associated with the development of malignant edema, malignant infarction, hemorrhagic transformation, and poor clinical outcomes.12⇓⇓-15
We hypothesized that besides quantifying infarction by spatial extension (ASPECTS), NWU could provide a second imaging dimension for characterizing the pathophysiology of ischemic lesions16 and predict the clinical outcome of individual patients with AIS. We tested this hypothesis by calculating the NWU using an automated ASPECTS-based method (ASPECTS-NWU) and investigating the performance of baseline ASPECTS-NWU as an imaging predictor of the 90-day functional outcome in patients with AIS and LVO. We also performed a subgroup analysis of patients with low ASPECTS (≤5).
MATERIALS AND METHODS
Patient Inclusion
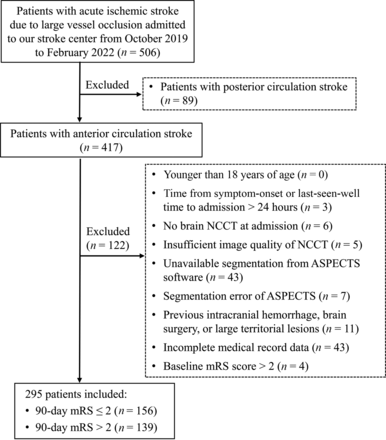
This retrospective study was reviewed and approved by the local institutional review board. The need to obtain patient informed consent was waived. From October 2019 to January 2022, consecutive patients with AIS due to LVO admitted to our stroke center were retrospectively reviewed. The detailed inclusion criteria were as follows: 1) patients older than 18 years of age; 2) AIS due to LVO of the ICA and/or the M1 or M2 segment of the MCA; 3) stroke-onset time or last-seen-well time to admission of ≤24 hours; 4) NCCT performed at admission; and 5) good image quality of NCCT without significant motion artifacts. The exclusion criteria were as follows: 1) patients with posterior circulation stroke; 2) unavailable segmentation from ASPECTS software; 3) segmentation error of ASPECTS software due to midline deviation or incorrect posture; 4) previous intracranial hemorrhage, brain surgery, or large territorial lesion; 5) incomplete medical records of clinical characteristics; and 6) baseline mRS score of >2.
Clinical Characteristics
The clinical characteristics were obtained from the electronic medical records, including demographic information (age, sex) and stroke and treatment characteristics, including stroke-onset or last-seen-well time to admission time, site of large-artery occlusion, NIHSS score at admission (NIHSSadmission), treatment methods (intravenous thrombolysis, mechanical thrombectomy, bridging therapy, and standard medical therapy), and vessel recanalization. The mRS scores at 90 days were dichotomized into favorable outcome (mRS score 0–2, no or slight disability and able to perform complex activities of daily living) and unfavorable outcomes (mRS score 3–4, disabled and dependent on others for activities of daily living; mRS 5–6, bedridden or dead).
Image Acquisition and Protocols
All patients with AIS underwent stroke protocol imaging on a 128-section multidetector CT scanner (Optima CT660; GE Healthcare) with the following parameters–NCCT: collimation, 16 × 1.25 mm; rotation time, 1.0 seconds; FOV, 250 mm; tube voltage, 120 kV(peak); tube current, 250 mAs; and 5.0-mm section reconstruction. CTP images were obtained using a periodic spiral approach (4D adaptive spiral mode; 100 kV[p]; 200 mAs; rotation time, 0.4 seconds; and pitch value, 0.984). The protocol included 30 consecutive spiral scans of the brain (z-axis, 80 mm; 2-second delay; and 1.7-second temporal resolution) after injection of 50 mL of contrast medium (iopromide, Ultravist 370; Bayer Schering Pharma) at a flow rate of 5 mL/s followed by administration of 30 mL of saline. The peak arterial phase of CTP data was selected on the basis of the arterial input function curve, and single-phase CTA was reconstructed with a section thickness of 0.625 mm for every 1 mm.
Image Analysis
Calculation of ASPECTS-NWU.
An automated software tool (Rapid ASPECTS, iSchemaView) was used to calculate an ASPECTS as well as the ASPECTS-NWU. The software performs a series of operations to generate an automated ASPECTS evaluation, which has been described in detail previously.17 The mean Hounsfield unit (HU) value was calculated for each predefined ASPECTS region. Each ASPECTS region was classified as either normal or abnormal using a machine learning–based algorithm. ASPECTS regions involved in the ischemic brain lesion were demonstrated in red on the output map. Then, the relative difference in mean HU values between each affected ASPECTS region in the ischemic hemisphere (HUischemic) and the contralateral normal hemisphere (HUnormal) was computed and presented as the percentage HU difference using the following formula:18
ASPECTS-NWU (%) = [1 − (HUischemic/HUnormal)] × 100%.
All affected ASPECTS regions were verified by a senior neuroradiologist (with 26 years of neuroradiology experience) to avoid inaccurate segmentation or identification of the ASPECTS regions.
Evaluation of Vessel Recanalization.
For the patients who underwent MT, the extent of recanalization was evaluated according to the modified TICI (mTICI) scale on the basis of postprocedural DSA by a neurointerventionalist (with 13 years of experience). Good recanalization was defined as an mTICI score of ≥2b. For patients who underwent only intravenous thrombolysis or standard medical therapy, vessel recanalization was evaluated on the basis of the follow-up CTA or MRA by a neuroradiologist (with 10 years of experience) who was blinded to all the clinical information.
Statistical Analysis
Continuous variables were presented as means (SD) or medians with an interquartile range (IQR) of the 25th–75th percentile. Categoric data were presented as numbers and corresponding percentages. The Kolmogorov-Smirnov test was used to assess the normality of data distribution. The correlation between ASPECTS-NWU and stroke-onset time was evaluated using a linear relation and a nonlinear logarithmic relation. Differences in clinical and imaging characteristics between patients with favorable and unfavorable outcomes were compared using the independent t test or Mann-Whitney U test for continuous variables and the χ2 test or Fisher exact test for categoric variables, as appropriate. Variables with significant P values (P < .05) on univariable analysis were entered into the multivariable logistic regression analysis.
Multivariable analysis was performed using the backward method to identify the independent predictors of a 90-day favorable outcome (mRS score, ≤2). Odds ratios with 95% confidence intervals were calculated. Receiver operating characteristic (ROC) curve analysis was used to assess the performance of different clinical and/or imaging models for predicting a favorable outcome at 90 days. The Youden index was used to calculate the optimal cutoff point, and the sensitivity and specificity were calculated on the basis of the best cutoff values. All tests were 2-tailed, and α was set at the .05 level. The statistical analyses were performed using the commercial software SPSS (Version 20.0; IBM) and MedCalc for Windows (Version 12.3.0; MedCalc Software).
RESULTS
Patient Characteristics
A total of 295 patients (171 men; mean age, 71.0 years) who fulfilled the inclusion criteria were included. The flowchart of patient selection is shown in Fig 1. There were 186 and 109 patients within the 6-hour and 6- to 24-hour time windows, respectively. The median stroke-onset time was 270 minutes (IQR, 143–432 minutes). The median ASPECTS and ASPECTS-NWU was 7 (IQR, 6–9) and 6.83% (IQR, 5.04%–8.76%). These patients had large-artery occlusions involving the MCA M1 segment (n = 196), MCA M2 segment (n = 54), ICA (n = 16), or ICA combined with the MCA M1 (n = 29). In total, 216 patients (73.3%) underwent MT. Vessel recanalization was achieved in 184 of 295 (62.4%) patients.
Flow chart for patient selection.
Favorable outcomes at 90 days were observed in 156 patients (52.9%). The univariable analyses showed that patients with favorable outcomes were younger (69.0 versus 73.0 years) and had lower NIHSS scores (11 versus 16), lower ASPECTS-NWU (5.73% versus 8.00%), higher ASPECTS (8 versus 6) at admission, and a higher percentage of vessel recanalization after therapy (75.6% versus 47.5%) compared with those with unfavorable outcomes (all P < .001). The detailed clinical and imaging characteristics stratified by 90-day functional outcomes are listed in the Online Supplemental Data.
Association between ASPECTS-NWU and Prognosis Stratified by Time Window
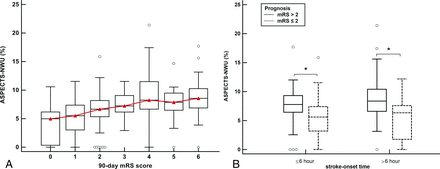
A significant nonlinear logarithmic correlation was identified among all patients between ASPECTS-NWU and stroke-onset time (r = 0.139, P = .017). ASPECTS-NWU significantly correlated with the mRS scores at 90 days (r = 0.451, P < .001) as shown in Fig 2A. A lower ASPECTS-NWU was more consistently observed in patients with good prognoses than in those with poor prognoses, regardless of the time window (both, P < .001; Fig 2B).
A, Differences in ASPECTS-NWU between patients with an mRS score of 0–6 at 90 days. The value of ASPECTS-NWU significantly correlates with the 90-day mRS scores (r = 0.451, P < .001). B, Boxplots display differences in ASPECTS-NWU between patients with favorable prognosis (mRS ≤2) and patients with poor prognosis (mRS > 2). Significantly lower values of ASPECTS-NWU can be observed in patients with good prognosis when compared to those with poor prognosis, regardless of the 6-hour time window. The asterisks indicate P < .001.
Independent Predictors of Favorable Outcome
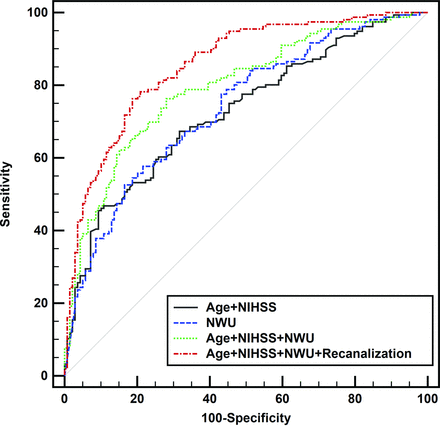
Multivariable logistic regression using backward selection revealed that age (OR, 0.96; 95% CI, 0.94–0.99; P = .007), NIHSSadmission (OR, 0.91; 95% CI, 0.87–0.96; P < .001), ASPECTS-NWU (OR, 0.79; 95% CI, 0.70–0.90; P < .001), and vessel recanalization (OR, 7.78; 95% CI, 3.96–15.29; P < .001) were independently associated with a favorable outcome at 90 days (Table 1). The area under the ROC curve of ASPECTS-NWU for the prediction of favorable outcomes was 0.737 (95% CI, 0.681–0.793), with a sensitivity of 57.7% and specificity of 78.4% at the optimal cutoff value of 6.32%. When ASPECTS-NWU was combined with the clinical model (NIHSSadmission and age), the area under the curve (AUC) was significantly increased to 0.794 (95% CI, 0.744–0.845; sensitivity, 76.3%; specificity, 71.9%; P < .001). When vessel recanalization was added, the AUC was further improved to 0.856 (95% CI, 0.814–0.899; sensitivity, 76.3%; specificity, 81.3%; P < .001; Table 2 and Fig 3). Two representative cases are shown in the Online Supplemental Data.
ROC curves for ASPECTS-NWU, age, and NIHSSadmission and their combinations with vessel recanalization for predicting a favorable prognosis (mRS ≤ 2) at 90 days. The discrimination power is significantly higher when ASPECTS-NWU is combined with the clinical model (age and NIHSS) and is further improved when vessel recanalization is added.
Independent clinical and imaging predictors for a 90-day favorable outcome
Predication models for a 90-day favorable outcomea
Subgroup Analysis for Patients with Low ASPECTS (≤5)
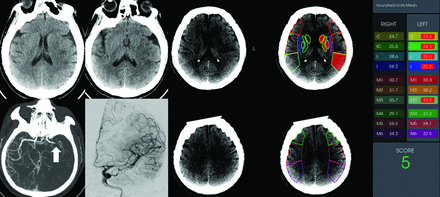
In the subgroup of patients with low ASPECTS of ≤5 (n = 68), 52 (76.5%) patients underwent MT, and 18 (34.6%) achieved a good neurologic outcome at 90 days. Lower mean ASPECTS-NWU values (8.44 [SD, 1.44]) were observed in patients with a good outcome compared with those with a poor outcome (10.15 [SD, 3.12]; P = .003). ASPECTS-NWU was consistently an independent negative predictor (OR, 0.56; 95% CI, 0.34–0.92; P = .022) of the 90-day neurologic outcome (Online Supplemental Data). The AUC of ASPECTS-NWU to predict a favorable outcome was 0.673 (95% CI, 0.542–0.805), with a sensitivity of 88.9% and a specificity of 48.0% at the optimal cutoff value of 9.86% in patients with low ASPECTS. A representative case is shown in Fig 4.
A patient with acute ischemic stroke due to occlusion of the M1 segment of the left MCA. This patient had an NIHSS score of 15 at admission and a low ASPECTS of 5. The mean value of ASPECTS-NWU at admission CT was 8.2%. Complete revascularization (mTICI grade 3) was achieved after mechanical thrombectomy. This patient had a favorable outcome, with an mRS score of 1 at 90 days. C indicates caudate nucleus; IC, internal capsule; L, lenticular nucleus; I, insula.
DISCUSSION
In the current study, we investigated the utility of ASPECTS-NWU as a quantitative imaging marker of 90-day clinical outcome in patients with AIS with LVO. Our results revealed that a lower ASPECTS-NWU at admission was consistently associated with a good prognosis at 90 days, regardless of the stroke-onset time. ASPECTS-NWU was an independent predictor of neurologic outcomes at 90 days, even in the subgroup of patients with a low ASPECTS of ≤5. The combination of ASPECTS-NWU and clinical variables yielded a good performance for stratifying the neurologic outcome at 90 days.
The efficacy of treatment on functional outcome in AIS is highly time-dependent. The concepts of ischemic core and penumbra have long been considered the key decision driver for AIS treatment and were used to guide patient selection in the extended time window.3 However, considering the differences in patients and tissue characteristics, it seems neither reliable nor reasonable to apply a single universal CTP core threshold across all time points from stroke onset.5,19 Some researchers have suggested that the threshold-derived CTP may overestimate the ischemic core (ghost core), especially in the early time window, which may potentially deny treatment to patients who might still benefit from reperfusion.6,7,20 Moreover, the software for CTP processing is not available in many centers, even comprehensive stroke centers. Therefore, it is desirable to develop practical and easy-to-implement imaging biomarkers that correlate with the time-dependent tissue viability and functional outcome after treatment.
Recently, NWU has been described as a quantitative imaging biomarker of ischemic lesion edema on NCCT and an individual indicator of tissue clock in patients with AIS.12,21,22 An elevated NWU in ischemic lesions indicates more severe ischemic damage to brain tissue.13,23,24 Nawabi et al12 reported that early elevated lesion water uptake in acute stroke predicted a poor outcome despite successful recanalization in a patient group with a stroke-onset time of <6 hours. Broocks et al25 also found that NWU and NIHSS were independently associated with the outcomes after adjusting for the degree of recanalization and ASPECTS for patients in the extended time window. In line with their findings, we found that the level of ASPECTS-NWU in ischemic lesions may mediate treatment effects and was independently associated with functional outcome, in addition to the traditional clinical variables (such as age and NIHSS). A combination of ASPECTS-NWU with NIHSSadmission, age, and vessel recanalization could achieve an AUC of 0.856, sensitivity of 76.3%, and specificity of 81.3% for the outcome stratification. Our sample size was larger than in previous studies. Moreover, the calculation of ASPECTS-NWU was based on an automated software-based analysis without the requirement of CTP, which may be a simple, rapid, and practical method for clinical use.
Individual variability exists in edema progression after stroke onset, especially among patients in the extended time window. Several previous studies have confirmed that ASPECTS-NWU may serve as a reliable indicator of lesion age in acute stroke18,26,27, of which a linear correlation between stroke onset time and ASPECTS-NWU was reported by Cheng et al.18 Consistent with their results, we observed a lower ASPECTS-NWU in patients with a stroke-onset time of ≤ 6 hours compared with those in the extended time window. However, in contrast to their results, we found a significant nonlinear logarithmic correlation between ASPECTS-NWU and stroke-onset time, which was in accordance with the study by Broocks et al.13,28 This nonlinear correlation may be a strength of NWU versus the real “time clock,” considering its quantitative nature. We performed a stratified analysis of the association between ASPECTS-NWU and prognosis according to the stroke-onset time. A higher ASPECTS-NWU was more consistently observed in patients with a poor prognosis than those with a good prognosis, regardless of the time window. Therefore, NWU provides an individualized estimate of the ischemic pathophysiology, the real tissue clock, which directly relates to the functional outcomes after treatment.
Several studies have emphasized the potential to expand the indicators of AIS treatment for patients in the extended window using the baseline ASPECTS on NCCT.8,10,29 Patients with an unfavorable prognosis usually have lower baseline ASPECTS (<6).30 In our study, not surprisingly, a lower ASPECTS was associated with an unfavorable prognosis. However, when NWU was added to the multivariable logistic regression analyses, ASPECTS was not an independent predictor of functional outcome. ASPECTS can only quantify the spatial extension and volume of ischemic lesions, whereas NWU additionally provides an imaging dimension for characterizing ischemic lesion pathophysiology and identifying the dynamic evolution of individual ischemic edema. Therefore, NWU may be more suitable for stratifying patient outcomes. The results from a subgroup analysis for patients with a low ASPECTS (n = 68) further supported this point of view. Patients with low ASPECTS (≤ 5) are generally considered unsuitable for endovascular therapy.3 However, favorable outcomes were achieved in 18 (34.6%) of the 52 patients who had successful revascularization in our study. Lower ASPECTS-NWU was the only independent imaging predictor of a favorable outcome at 90 days. Several recent studies have reported a benefit of MT in carefully selected patients with low ASPECTS.31,32 In accordance with the previous studies, our findings suggest that this subset of patients should not be entirely excluded from endovascular treatment. In our study, a simple NCCT-based paradigm may allow the selection of patients with a large infarct core.
There were several limitations to this study. First, despite its promise in quantifying the evolution and severity of brain edema, challenges still existed in the measurements of NWU values. The ASPECTS-based approach in our study may obviate the need for CTP but may rely on the presence of early ischemic changes. Careful verification of all affected ASPECTS regions by experienced doctors is necessary. Moreover, the affected ASPECTS regions might only be ischemic for part of the total defined region, which may consequently lead to an underestimation of the true NWU. Therefore, the median ASPECTS-NWU in our study (6.83%) was lower than the previously reported NWU measure based on CTP. An accurate means of automatically extracting infarct regions and measuring NWU on the basis of NCCT is warranted and would be of benefit for standardizing NWU measurement in large-cohort studies and clinical trials of stroke. Second, because we enrolled patients who received only standard medical treatment without MT, vessel recanalization of these patients could only be assessed by follow-up CTA or MRA. However, most (73.2%) of our patients underwent MT, and vessel recanalization was assessed according to the mTICI grade on DSA. Finally, this was a retrospective, single-center study; therefore, the results may not be generalized to all patients with stroke. In addition, the number of patients with a low ASPECTS was relatively small. Further prospective studies are warranted to investigate the generalizability of our results and the utility of NWU for the selection of patients for treatment, especially those with a large ischemic core at admission.
CONCLUSIONS
The simplified ASPECTS-NWU could provide pathophysiologic information about individual ischemic lesions and could be correlated with the 90-day neurologic outcomes, regardless of the stroke-onset time, in patients with AIS and LVO. A lower ASPECTS-NWU may independently predict favorable neurologic outcomes at 90 days, even in the subgroup of patients with low ASPECTS (≤5). Integrating ASPECTS-NWU with the clinical models could improve the efficiency of outcome stratification.
Footnotes
Drs Shan-Shan Lu and Rong-Rong Wu contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (grant No. 82171907 to Shan-Shan Lu and grant No. 81971613 to Hai-Bin Shi).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received August 23, 2022.
- Accepted after revision November 11, 2022.
- © 2023 by American Journal of Neuroradiology